Ralph O. Allen
Trace Analysis: Environmental, Archaeological, and Forensic Applications
Professor Allen is not currently accepting graduate students.
The development of sensitive analytical methods has helped provide more detailed information about the small chemical differences between materials which seem similar but have different histories. In some cases, like geological samples, the large scale geochemical processes can give rise to subtle yet understandable differences in those elements which are present at trace levels. We have used instrumental neutron activation analysis and X-ray fluorescence to study several types of geological materials. These materials have been investigated as part of an ongoing effort to interpret the migration of trace elements during geochemical processes. In some cases, these same geological materials have been used by prehistoric humans and hence our studies have had archaeological implications.
To provide accurate trace element data on large numbers of samples, we have investigated several other analytical methods. The inductively coupled plasma emission source used to provide ions for a quadrupole mass spectrometer (ICP-MS) holds considerable promise. This method is also being used to study soil and glass samples for applicability in the forensic laboratory. Our efforts in the forensic science field are part of a continuing cooperative effort with scientists at the FBI’s research facilities. In the past, traces of explosive residues and epoxies have been analyzed to differentiate the sources of the materials. The latest forensic science efforts have been directed toward the important new area of DNA “fingerprinting.” This major forensic tool allows small amounts of genetic material to be used to match the DNA from a suspect.
Representative Publications
Forensic determination of ricin and the alkaloid marker ricinine from castor bean extracts. Darby SM, Miller ML, Allen RO. J Forensic Sci. 6, 1033-42 (2001).
A mass spectrometric method for quantitation of intact insulin in blood samples. Darby SM, Miller ML, Allen RO, LeBeau M. J Anal Toxicol. 25, 8-14 (2001).
Analysis of two multiplexed short tandem repeat systems using capillary electrophoresis with multiwavelength fluorescence detection. Isenberg AR, Allen RO, Keys KM, Smerick JB, Budowle B, McCord BR. Electrophoresis. 19, 94-100 1998 .
Lester S. Andrews
Spectroscopy and Photochemistry of Matrix Isolated Species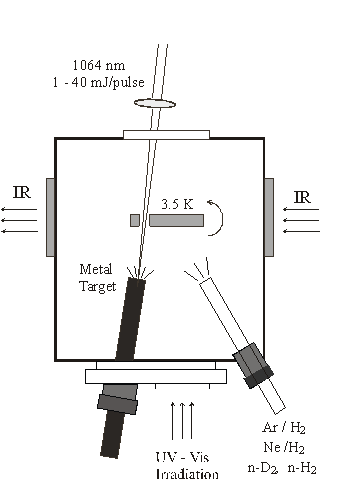
Normally inaccessible chemical species including free radicals, hydrogen-bonded complexes, unusual inorganic species, transition metal containing intermediates, metal hydrides, and molecular ions are under investigation using infrared matrix-isolation spectroscopy. These transient molecules are prepared by reactions of suitable atoms and/or molecules prediluted in argon by precursor photolysis, or by microwave discharge of the reagents during condensation at 7 K in a vacuum chamber. Solid argon isolates the reaction or photolysis products and preserves them for spectroscopic study at 7 K. Neon is used to isolate new reactionproducts on a 4 K substrate. Pure H2 and D2 are also be employed as a reagent and matrix for the investigation of novel metal hydrides. A new source of atoms that require high temperatures to generate, namely pulsed laser ablation from solid samples, has been developed for use in matrix-infrared spectroscopy in this laboratory. This method exploits two advantages: first, the ablated atoms contain excess energy which can activate reactions with small molecules, and second, collisions with matrix atoms during the condensation process relax energetic product molecules and allow them to be trapped in the solid matrix for spectroscopic study. In addition, laser-ablation also produces cations and electrons for reactions to make charged products.
The experimental apparatus used in hydrogen matrix investigations is sketched in the figure. Infrared spectra are recorded after co-depositing reagent and host matrix gases on the cold sample window and rotating by 90 degrees to face the infrared light beam.
These infrared spectroscopic matrix-isolation studies characterize the bonding and structure of chemical intermediates, interesting new inorganic molecules and complexes and molecular ions and often provide a useful complement and guide to high resolution gas phase work. Stable isotopes are used to determine assignments of the observed infrared absorptions to fundamental vibrational frequencies. Comparison of vibrational energies within related chemical species provides conclusions about the bonding of these newly observed chemical intermediates. Selection rules based upon molecular symmetry and vibrational analysis help determine the molecular geometry. Ab initio electronic structure calculations are done to find molecular structures compatible with the infrared spectrum. Agreement between calculated and observed isotopic vibrational spectra provides further evidence for the discovery of new transient molecular species.
Please click here for a full list of publications.

Robert F. Bryan
Robert Bryan was a faculty member of the U.Va. Chemistry Department for 36 years. During his career, Professor Bryan established an international reputation in crystallography and published more than 150 papers in the area. He was born in Rhu, Scotland in 1933, and earned his B.Sc. (1954) and Ph.D. (1957) from the University of Glasgow. His postdoctoral training was at ETH (BAttelle Fellow) and MIT (Alfred P. Sloane Fellow and a NIH Fellow) from 1957 – 1961. In 1961, he joined the faculty at Johns Hopkins University School of Medicine in the Biophysics Department. He joined U.Va. in 1967 as an Associate Professor of Chemistry and became a Full Professor in 1982. He held several offices in the international Union of Crystallography and was an editor of Acta Crystallographica and The Journal of Applied Crystallography. In addition to a very active research career (over 150 publications), Prof. Bryan was the Associate Dean for the Graduate School of Arts and Sciences from 1970 – 1973.

Robert G. Bryant
Nuclear Magnetic Resonance in Chemistry
The Bryant laboratory utilizes a wide range of nuclear and electron spin resonance methods to characterize intra and intermolecular dynamics. A primary focus is the rich information available from the study of the magnetic field dependence of the nuclear spin-lattice relaxation rate as a function of the magnetic field strength or what is called the magnetic relaxation dispersion (MRD). We have constructed unique instrumentation that permits acquisition of MRD profiles for a variety of nuclear resonances in high resolution. These measurements provide a direct measure of the time fluctuations in various nuclear or nuclear-electron dipolar couplings. The resulting MRD profile reports relative inter and intramolecular motions over the time range from about ten microseconds to one picosecond. We are developing methods for probing the fluctuation spectrum of specific vectors defined in structurally interesting proteins and are trying to understand how these fluctuations affect molecular function. In related work, we apply the nuclear spin relaxation induced by freely diffusing paramagnetic solutes in cosolutes to define how one molecule samples the local environment of another and explores the surface or even penetrates into a complex structure like a folded protein. The measurements are sensitive to relatively weak intermolecular interactions and permit definition of highly localized intermolecular free energy differences in solutions. Multinuclear studies of proteins in a number of dynamical environments provide a fundamental characterization of how the protein structure fluctuates in time and how energy is redistributed in the folded structure. The practical implications range from understanding protein catalytic function to developing new techniques for diagnostic medicine in the context of magnetic resonance imaging or MRI.
Recent Publications
Water-proton-spin-lattice-relaxation dispersion of paramagnetic protein solutions. Diakova G, Goddard Y, Korb JP, Bryant RG. J Magn Reson. 208:195-203 (2010).
Water and backbone dynamics in a hydrated protein. Diakova G, Goddard YA, Korb JP, Bryant RG. Biophys J. 98:138-46 (2010).
Dynamics of water in and around proteins characterized by 1H Spin-Lattice Relaxometry. Bryant RG. Comptes Rendus Physique. 11(2), 128-135(2010).
Dimensionality of diffusive exploration at the protein interface in solution. Grebenkov DS, Goddard YA, Diakova G, Korb JP, Bryant RG. J Phys Chem B. 113, 13347-13356 (2009).
Water molecule contributions to proton spin-lattice relaxation in rotationally immobilized proteins. Goddard YA, Korb JP, Bryant RG. J Magn Reson. 199, 68-74 (2009).

Francis A. Carey
Francis A. Carey is a native of Pennsylvania, educated in the public schools of Philadelphia, at Drexel University (B.S. in chemistry, 1959), and at Penn State (Ph.D. 1963). Following postdoctoral work at Harvard and military service, he was appointed to the chemistry faculty of the University of Virginia in 1966. Prior to retiring in 2000, he regularly taught the two-semester lecture courses in general chemistry and organic chemistry. With his students, Professor Carey has published over forty research papers in synthetic and mechanistic organic chemistry. In addition to this text, he is coauthor (with Robert C. Atkins) of Organic Chemistry: A Brief Course and (with Richard J. Sundberg) of Advanced Organic Chemistry, a two-volume treatment designed for graduate students and advanced undergraduates. He was a member of the Committee of Examiners of The Graduate Record Examination in Chemistry from 1993-2000.
James N. Demas
Photochemistry and Photophysics of Transition Metal Complexes
Professor Demas is not currently accepting graduate students.
Molecules excited by light lose energy by emission of light, transfer of energy or an electron to other molecules, photochemistry, and non-destructive radiationless processes. Solar energy conversion, chemical analysis, and light intensity measurements require information from the study of these processes. The processes can be highly sensitive to environmental factors such as solvent and interactions with organized media such as micelles, cyclodextrins, membranes, proteins, and DNAs. In addition, luminescence properties are very sensitive to the environment in polymer-supported sensors. We are elucidating the nature of these processes, correlating properties with molecular structure and environment, and developing new chemical, instrumental, and mathematical tools for studying these processes.
Our work includes:
- Design, synthesis and characterization of highly luminescent Os, Ir, Re, and Ru complexes.
- Evaluating photochemical properties, excited state ordering, and paths of energy loss.
- Fundamental and applied studies of interactions of photosensitizers with polymers, micelles, membranes and other organized media.
- Developing new luminescence-based sensors (e.g., oxygen, pH, metal ion).
- Design and utilization of metal complexes as probes of the structure and dynamics of organized media such as DNAs and membranes.
- Instrumental and theoretical developments in ultrasensitive, multicomponent fluorometric analyses.
An example of an analytical sensor is shown in the figure.
The photoluminescence of a tris(4,7-diphenyl-1,10-phenanthroline)ruthenium (II) complex in a polymer film is shown while the film is being breathed over. The luminescence is quite sensitive to deactivation by oxygen, and the luminescence intensity is a direct measure of the oxygen in the subject’s breath. Less oxygen yields more luminescence. The region immediately after the subject held his breath is revealing.
Recent Publications
Aromatic difluoroboron β-diketonate complexes: effects of π-conjugation and media on optical properties. Xu S, Evans RE, Liu T, Zhang G, Demas JN, Trindle CO, Fraser CL. Inorg Chem. 52:3597-610 (2013).
Viscosity and temperature effects on the rate of oxygen quenching of tris-(2,2′-bipyridine)ruthenium(II). Reynolds EW, Demas JN, DeGraff BA. J Fluoresc. 23:237-41 (2013).
Environmental sensitivity of Ru(II) complexes: the role of the accessory ligands. Dixon EN, Snow MZ, Bon JL, Whitehurst AM, DeGraff BA, Trindle C, Demas JN. Inorg Chem. 51:3355-65 (2012).
Photophysical and analyte sensing properties of cyclometalated Ir(III) complexes. Leavens BB, Trindle CO, Sabat M, Altun Z, Demas JN, DeGraff BA. J Fluoresc. 22:163-74 (2012).
Laser phosphoroscope and applications to room-temperature phosphorescence. Payne SJ, Zhang G, Demas JN, Fraser CL, Degraff BA. Appl Spectrosc. 65:1321-4 (2011).

Cassandra L. Fraser
Difluoroboron dibenzoylmethane-poly(lactic acid) analogues exhibit both intense fluorescence and long-lived room temperature phosphorescence. When fabricated as nanoparticles, these simple, dual-emissive biomaterials serve as optical oxygen probes for biology and medicine, with impressive combined spatial and temporal resolution. Along with fundamental studies, the materials have been optimized with respect to fluorescence and phosphorescence emission colors, relative intensities, luminescence lifetimes, oxygen sensitivities, and fabrication. With collaborators, we developed a portable, cost-effective, laptop camera imaging system that, in conjunction with boron nanosensors, allows for dynamic, real-time, single or dual mode (ratiometric and/or lifetime) tissue oxygen imaging. We have demonstrated the utility of these materials for in vitro and in vivo optical oxygen imaging in cell, tumor, wound, vascular, brain, immunological, tissue engineering, and other contexts.
Mechanochromic Luminescence and Other Stimuli Responsive and Environment Sensitive Properties
Difluoroboron β-diketonate dyes also show surprising properties as molecular solids. For example, we showed that the difluoroboron complex of avobenzone, a simple sunscreen ingredient, has narrow bandwidth green, cyan, or blue emission depending on the solid form. Furthermore, the emission color changes when crystals are crushed or films are scratched or rubbed. Surprisingly, for thin films, the mechanochromic luminescence is reversible. For the avobenzone complex, regions where force is applied turn yellow but return to the original green-blue background color within minutes at room temperature or seconds with heating. The writing-fading process may be repeated many times. Emission colors, force responsiveness, and self-healing times may be tuned through molecular design, and self-erasing properties may be monitored with video camera imaging. These simple Scratch the Surface InksTM show promise as mechanical sensors and renewable inks for rewritable surfaces. They have even inspired creative works in music, art, and design. Other interesting properties of boron dyes, and in some cases even β-diketones absent difluoroboron, include solvatochromism, viscochromism, halochromism, aggregation induced emission, dye thickness and loading effects, and energy transfer in dye mixtures. Interestingly, some dyes are also thermally responsive and form supercooled liquids. Synthesizing new dyes, exploring their many fascinating properties, and tailoring materials for imaging and sensing in biology, medicine, and other contexts serves as the focus of our research.
Integrative Interdisciplinary Projects
Professor Fraser also has a great passion for envisioning and leading innovative interdisciplinary programs that integrate teaching, research, community engagement and creative pursuits. These projects are often inspired by materials, and environmental health and sustainability themes. They build bridges across STEM and non-STEM disciplines and engage students, faculty, and the community with thought leaders from across UVA and the globe. Examples include the UVA Page Barbour supported Transduction and Plastic/ity projects, the Carnegie Corporation funded Designing Matter Common Course, the NIH Global Health funded Metals in Medicine and the Environment, the Biomaterials Workshop, the Echols seminar Color: Across the Spectrum, and the Science, Careers and Society Forum. Professor Fraser often collaborates with artists and designers on exhibitions and performances (e.g. Chromogenic Materials, Agents of Architecture, UVA Music Technosonics Festival, UVA Art Bestiary Exchange Portfolio, Environmental Art Activism, and Time books). She also engages in design projects to establish new kinds of venues for conducting and displaying interdisciplinary work (e.g. WallSpace, Real World Chemistry Lab). New research is concerned with Anthrochemistry—chemistry of the Anthropocene, investigating the human impacts of chemistry through element, molecule, and material case studies via an interdisciplinary global systems chemistry approach. Of particular interest are ways that materials, their pathways, and processes, are mapped onto and into our bodies and intersect with our everyday lives, affecting health and wellbeing. Laws, policies, social and environmental justice, and ethics and responsibility are also considered. Creative interdisciplinary ways of communicating findings to both university and broader audiences are also of interest.
Selected Publications
Luminescent Difluoroboron β-Diketonate PLA-PEG Nanoparticles. Kerr, C.; DeRosa, C. A.; Daly, M. L.; Zhang, H.; Palmer, G. M.; Fraser, C. L. Biomacromolecules 2017, 18, 551-561.
Oxygen Sensing Difluoroboron β-Diketonate Polylactide Materials with Tunable Dynamic Ranges for Wound Imaging. DeRosa, C. A.; Seaman, S. A.; Mathew, A. S.; Gorick, C. M.; Fan, Z.; Demas, J. N.; Peirce, S. M.; Fraser, C. L. ACS Sensors 2016, 1, 1366-1373.
Mechanochromic Luminescence and Aggregation Induced Emission of Dinaphthoylmethane β-Diketones and their Boronated Counterparts. Butler, T.; Morris, W. A.; Samonina-Kosicka, J.; Fraser, C. L. ACS Appl. Mater. Interfaces 2016, 8, 1242-1251.
Polymorphism and Reversible Mechanochromic Luminescence for Solid-State Difluoroboron Avobenzone. Zhang, G.; Lu, J.; Sabat, M.; Fraser, C. L. J. Am. Chem. Soc. 2010, 132, 2160-2010.
A Dual-Emissive Materials Design Concept Enables Tumour Hypoxia Imaging. Zhang, G.; Palmer, G. M.; Dewhirst, M. W.; Fraser, C. L. Nat. Mater. 2009, 8, 747-751.
Multi-Emissive Difluoroboron Dibenzoylmethane Polylactide Exhibiting Intense Fluorescence and Oxygen-Sensitive Room-Temperature Phosphorescence. Zhang, G.; Chen, J.; Payne, S. J.; Kooi, S. E.; Demas, J. N.; Fraser, C. L. J. Am. Chem. Soc. 2007, 129, 8942-8943.
See more at Google Scholar: https://scholar.google.com/citations?user=gUlmJ6UAAAAJ&hl=en

Graeme Gerrans
Professor Gerrans is primarily engaged in teaching and chemical education. He has taught undergraduate chemistry at the University of the Witwatersrand, South Africa and here at the University of Virginia. He has held visiting professorships at the California Institute of Technology (1972), the University of Illinois (1979), the University of East Anglia, England (1986) and the University of Virginia (1990). Amongst the awards that he has received are the Convocation Distinguished Teacher’s Award (University of the Witwatersrand) and the Education Medal (South African Chemical Institute). He has published over 80 papers and is the co-author, together with P and R Hartmann-Petersen, of “Encyclopedia of Science and Technology,” (New Africa Books, 2007).
Most recently, Professor Gerrans was a faculty in the Spring 2009 UVA Semester at Sea Program.

Russell N. Grimes
Russ Grimes was raised in Pennsylvania and is a B.S. Chemistry alumnus of Lafayette College He received his Ph.D. in Chemistry from the University of Minnesota as a student of Professor William Lipscomb, although his thesis research was conducted at Harvard University where he was a Harvard Fellow. Following postdoctoral work at Harvard and with Professor M. F. Hawthorne at the University of California, Riverside, he was a faculty member in the Department of Chemistry from 1963 to 2003, becoming a Full Professor in 1973 and serving a term as Department Chair from 1981 to 1984. Over a 40-year period he and his students were among the pioneers in the synthesis and study of polyhedral molecular cage compounds of boron, along the way discovering metal-promoted oxidative cage fusion, tricarbon and large tetracarbon carboranes, stable triple-decker sandwich complexes (which were also the first electrically neutral triple-deckers), isolable tetra-, penta-, and hexadecker molecular sandwiches, linked multidecker complexes, polyhedral metallaboranes, and many novel clusters ranging from C2B3H7 to 14-vertex M2C4B8 systems. A major theme in their work was the development and exploration of small carborane ligands in the designed synthesis of novel transition-metal organometallic systems, especially those having electrically conducting or catalytic properties. The Grimes group together with William Hutton, in work initially reported in the Journal of the American Chemical Society in 1980 and 1981, developed the first successful 2D COSY (correlated spectroscopy) NMR technique involving a quadrupolar nucleus (11B) and in a 1984 JACS full paper detailed its use in both homonuclear 11B-11B) and heteronuclear (11B-1H) spectroscopy.
Professor Grimes was a Senior Fulbright Scholar at the University of Canterbury, New Zealand, in 1974-75, a Guest Professor at the University of Heidelberg, Germany, in 1986, a Humboldt Scholar there in 1997-98, and a Visiting Scholar at the Korea Advanced Institute for Science and Technology in 1997. He received a Boron U.S.A. (BUSA) Award for Distinguished Achievements in Boron Science in 1990 and was elected a Fellow of the American Association for the Advancement of Science in 1981. He served as an American Chemical Society Tour Speaker on six occasions. He is the author or co-author of 240+ research publications and the books Carboranes (Academic Press, 1970, Russian translation 1974) and Carboranes Second Edition, (Elsevier, 2011). He edited the book Metal Interactions with Boron Clusters (Plenum, 1982) and is Editor-in-Chief of Inorganic Syntheses, Vol. 29 (John Wiley, 1992). He has served on the editorial board of Inorganic Chemistry and the editorial review panel of Dalton Transactions, and is a past president of the Board of Directors of Inorganic Syntheses.
The Third Edition of Carboranes was published by Elsevier in August, just 5 years following the Second Edition. The book reflects the rapid pace of development in this field, which is generating hundreds of publications each year in areas as diverse as nanomaterials, pharmacology, diagnostic and therapeutic medicine, extraction of radioactive metals from nuclear waste, catalysis, and organic synthesis. Professor Grimes was recently honored by a special issue of the Journal of Organometallic Chemistry, featuring over 40 contributed papers from international scientists in recognition of his contributions to boron chemistry throughout his 50-year career.

Sidney Hecht
Sidney Hecht was the John W. Mallet Professor of Chemistry and a Professor of Biology at UVA from 1978 – 2008. During his thirty years he led a very productive research group in the areas of synthesis and mechanism of action of bleomycin family antitumor antibiotics, peptidyltransferase as a source of synthetic enzymes, mechanism of action/inhibition of mammalian DNA topoisomerase I, and inhibition of signal transduction at the level of p90RSK. He is the recipient of numerous awards icluding the Alfred P. Sloan Research Fellowship (1975-79), a National Institutes of Health Research Career Development Award (1975-80), a John Simon Guggenheim Memorial Fellowship (1977-78), the 1996 Arthur C. Cope Scholar Award, the Virginia’s Outstanding Scientist Award (1996), a 1998 Research Achievement Award, American Society of Pharmacognosy and in 2004 he was elected as a Fellow of the American Association for the Advancement of Science. In a career spanning more than three decades, Sidney Hecht has held both academic and industrial research positions. From 1981 to 1986 in addition to his professorship at UVA, he held concurrent appointments at Smith Kline & French Laboratories, first as Vice President Preclinical R&D, then Vice President Chemical R&D. He is currently Director of the Center for BioEnergetics in the Biodesign Institute and Professor of Chemistry at Arizona State University.
Eric Herbst
Professor Herbst’s major research field lies in the interdisciplinary area of molecular astronomy, which is the study of molecules throughout the universe, especially in regions in between stars known as interstellar clouds. These objects eventually collapse to form new generations of stars and planetary systems, so the molecules found in interstellar clouds are related to the molecules found in planets such as our own. Herbst is specifically interested in the chemical processes by which molecules grow, in using these chemical processes to predict the actual concentrations of molecules, and in the role of molecules in the understanding of their physical environments. His research was featured in Chemical and Engineering News, the popular journal of the American Chemical Society. A fellow of the American Physical Society and the Royal Society of Chemistry (U. K.), Herbst has won a number of international prizes including the Centenary Award of the Royal Society of Chemistry.
| Below are pictures of two astronomical objects where molecules are found. | |
| On the right is a nebula of gas and dust surrounding an old star. It is known as the “red rectangle.” |  |
| Below is an interstellar cloud so dense that no light can pass through it. | |
 |
|
Recent Publications
A New Model on the Chemistry of Ionizing Radiation in Solids: CIRIS,.Shingledecker, C. N., & Herbst, E., Phys. Chem. Chem. Phys., 19, 11043-11056 (2017)
Unified Microscopic-Macroscopic Monte Carlo Calculations of Complex Organic Molecule Chemistry in Cold Cores,. Chang Q., & Herbst, E., ApJ, 819:145(1-13) (2016)
Chemical and Physical Characterization of Collapsing Low-Mass Prestellar Dense Clouds, Hincelin, U., Commercon, B., Wakelam, BV., Hersant, F., Guilloteau, S., & Herbst, E., ApJ, 822:12(1-31) (2016)
Complex organic molecules in protoplanetary disks, Walsh, C., Millar, T. J., Nomura, H., Herbst, E., Widicus Weaver, S., Aikawa, Y., Laas, J. C., & Vasyunin, A. I., A&A, 563, A33(1-35) (2014)
Reactive Desorption and Radiative Association as Possible Drivers of Complex Molecule Formation in the Cold Interstellar Medium, Vasyunin, A. I., & Herbst, E., ApJ, 769, id. 34(1-9) (2013)
Donald F. Hunt
Analytical Biochemistry
The goal of our research is to develop new methods and instrumentation for the structural characterization of proteins and their post-translational modifications at the low femtomole/attomole level and to apply these new methods to important structural problems in cell biology and immunology. Towards this end, we have pioneered the use of nanoflow HPLC in conjunction with microelectrospray ionization on ion trap and Fourier transform mass spectrometers. Briefly stated, the approach involves the use of proteolytic enzymes to convert the protein or group of proteins into a complex mixture of peptides, which are then fractionated by nanoflow-HPLC and eluted directly into the mass spectrometer. Mixtures containing thousands of different peptides can be analyzed in this manner. Protonated peptides of a particular mass are selected under computer control of the instrument, fragmented on collision with helium atoms and the resulting fragments are then separated and mass analyzed. Dissociation of the peptide ions occurs more or less randomly at each of the amide bonds in the molecules to produce a collection of fragments. The mass difference between two fragments differing by a single amino acid defines the mass and thus the identity of the extra residue in the longer fragment. Peptide sequence analysis is performed routinely at the femtomole and low attomole levels on the ion trap and Fourier transform instruments, respectively. Mass spectra acquired in the above manner can also be used to search databases and to identify known proteins. Currently, this approach is the most sensitive method in the world for protein characterization.
Our research focuses on two major applications of the above technology. The first involves identifying peptides that trigger the immune system to kill diseased cells. Cytotoxic T lymphocytes (CTL) or killer cells are an arm of the immune system concerned with recognition of cells that express new antigens, proteins, as a result of viral infection or cellular transformation (cancer). Cells convey their health status to the immune system by generating fragments from each of the approximately 10,000 proteins being synthesized, loading them onto a protein carrier (MHC molecule), and transporting them to the cell surface for screening by the killer cells. CTL lyse those cells that display new fragments, antigens that are associated with a particular disease state. Identification of these antigens is the first step in the preparation of vaccines that promote immunity against the above diseases. Peptides that cause the immune system to kill melanoma and lung cancer cells, to reject bone marrow transplants to leukemia patients, and to lyse tuberculosis infected cells have been identified in the laboratory recently. Efforts are in progress to characterize the peptide antigens that (a) cause rejection of tissue transplants, (b) trigger organ or tissue destruction in such autoimmune disorders as diabetes, arthritis, and multiple sclerosis, and (c) initiate an immunological response to breast, ovarian, colorectal, lung, and prostate cancers.
The second application involves research in the field of proteomics. DNA sequence information on the human genome and that of selected organisms is now becoming available at an ever-increasing rate and will provide the starting point for the development of novel therapeutic interventions against many of the world’s diseases. The next challenge is at the level of proteomics, understanding the functions of proteins encoded by a particular genome. Presently under development are mass spectrometry methods that will facilitate differential display and quantitation of most, if not all proteins expressed by healthy vs diseased cells or cells grown in the presence or absence of drugs or other agonists. Mass spectrometry is also being used to analyze all proteins secreted by a particular cell type, to identify components of functionally active protein complexes, to probe protein-protein and protein-DNA interactions, and to locate post-translational modifications and covalently attached ligands. Recently, we have developed methods that facilitate analysis of all phosphoproteins expressed in a particular cell population.
Recent Publications
Peptide Binding Motifs of Two Common Equine Class I MHC Molecules in Thoroughbred Horses, Bergman T, Lindvall M, Moore E, Sidney J, Miller D, Talmadge R, Myers P, Shabanowitz J, Osterreider N, Peters B, Hunt DF, Antczak DF, Sette A, Immunogenetics, 2017 May; 69(5):351-358. PMCID:PMC 5555743.
Canonical and Cross–reactive Binding of NK Cell Inhibitory Receptors to HLA-C Allotypes is Dictated by Peptides Bound to HLA-C. Sim MJ, Malaker SA, Khan A, Stowell JM, Shabanowitz J, Peterson ME, Rajagopalan S, Hunt DF, Altmann DM, Long EO, Boyton RJ, Front Immunol 2017 Mar 14(8);193. Doi:10.3389/fimmu.2017.00193.eCollection 2017 PMCID:PMC 5348643.
The Antigenic Identify of Human Class I MHC Phosphopeptides is Critically Dependent Upon Phosphorylation Status, Mohammed F, Stones DH, Zarling AL, Willcox CR, Shabanowitz J, Cummings KL, Hunt DF, Cobbold M, Engelhard VH, Willcox BE, Oncotarget J, 2017; April 8 (33):54160-54172. PMID: 28903331.
Front-End Electron Transfer Dissociation Coupled to a 21 Tesla FT-ICR Mass Spectrometer for Intact Protein MS/MS Analysis, Weisbrod DR, Kaiser NK, Early L, Mullen C, Syka JEP, Dunyach JJ, Anderson LC, English AM, Blakney GT, Shabanowitz J, Hendrickson CL, Marshall AG, Hunt DF, J Amer Soc Mass Spectrom, 2017;Jul 18: PMID 28721671.
Shared Peptide Binding Specificities of HLA Class I and Class II Alleles Associate with Cutaneous Nevirapine Hypersensitivity and Identify Novel Risk Alleles, Pavlos R, McKinnon EJ, Ostrov DA, Peters B, Buus S, Koelle D, Chopra A, Rive C, Redwood A, Restrepo S, Bracey A, Kaever T, Myers, P, Speers E, Malaker SA, Shabanowitz J, Jing Y, Gaudieri S, Hunt DF, Carrington M, Haas DW, Mallal S, Phillips EJ, Sci Rep, 2017; 7(1):8653.doi:10.1038/s41598-017-08876-0. PMID: 28819312.

Timothy L. Macdonald
Bioorganic and Synthetic Organic Chemistry
The unifying theme of my research program is the application of organic chemical theory and technique to the investigation of problems in biology. Our longstanding interest has been in elucidating the molecular interactions and mechanisms of small molecule-protein interactions. Such interactions are fundamental to physiology, pharmacology, and toxicology and understanding these processes in molecular terms is essential for predicting modes of metabolism and developing new drugs. Our efforts are currently directed at several rather broad areas: elucidation of the molecular pharmacology of ligand-receptor interactions; identification of the molecular processes underlying idiosyncratic drug reactions; determination of the chemical mechanisms of the oxidative transformation of foreign and endogenous compounds by monooxygenase and dioxygenase enzymes; and characterization of the molecular mechanisms underlying the neurotoxicity of small molecules, metal ions, and reactive oxygen species.
Current studies of ligand-receptor interactions include the lysophospholipid signaling system, the colchicinoid site on tubulin, and the ternary drug-DNA-topoisomerase II complex responsible for the cytotoxicity of this class of anticancer agents. Lipid phosphoric acid mediators, such as lysophosphatidic acid and sphingosine-1-phosphate, have a range of biological properties mediated through at least eight G-protein coupled receptors of the EDG family. The cellular biology of these receptors and the physiological mechanisms controlling the processing of the lysophospholipids is beginning to emerge. We are undertaking a set of investigations directed at elucidating the molecular pharmacology of this receptor family and at utilizing this knowledge to target the modulation of lysophospholipid signaling to treat human disease. Specifically, our studies of the lysophospholipid autocoids have been directed at identifying the molecular determinants of the lipid mediator and the receptor that have been proposed to be critical to activity and at developing receptor selective agents for the EDG receptors.
We also have a long-standing interest in understanding ligand-receptor interactions which form ternary DNA-drug complexes with a DNA processing enzyme, and a small molecular ligand. Such complexes are a common theme for a diverse variety of “effectors” of cellular function, including hormone regulators of cellular transcription and translation, and of many antineoplastic agents. We have been examining the interaction of the representative antitumor agent, etoposide, which forms a ternary DNA-drug complexes with DNA topoisomerase I. Another area of research involves elucidation of the molecular mechanism through which colchicine interacts with its target, tubulin. These programs have fully integrated synthetic studies of rationally designed molecules and mechanistic studies of the processes through which these agents interact with their targets. Our studies will advance the knowledge of antitumor drug development for these classes of agents and culminate in the design and synthesis of fundamentally new structural classes of inhibitors of these critical anticancer targets.
An additional area of research is the molecular and cellular mechanisms by which idiosyncratic drug reactions occur. These reactions are the source of a range of toxic reactions to therapeutic agents and are thought to be mediated through the confluence of risk factors associated with drug bioactivation to reactive species, the formation of specific protein antigens and subsequent immune response. A knowledge of the chemical and immunological basis for idiosyncratic reactions would enable a protocol for prospectively identifying individual patient risk or susceptibility to particular agents or drug classes. We have previously investigated the mechanism of blepharoconjunctivitis and dermatitis associated with the use of the antiglaucoma drug, apraclonidine. Our current focus is on the mechanism underlying the aplastic anemia associated with the use of the anti-epileptic agent, felbamate.
Our research is additionally exploring the mechanistic and etiologic relationships between neurotoxicity and aberrant CNS processing of foreign and endogenous agents. The brain is the site of multiple, chronically progressive and clinically devastating diseases in which specific neuronal populations undergo neurodegeneration. An environmental contribution has been implicated in the etiology of many of these diseases, including Parkinson’s disease, motoneuron disease, and Alzheimer’s disease. In one avenue of study, we are examining the potential for the formation of endogenous or exogenous toxins by oxidative enzymes that have been localized to particular neuronal populations, such as the cytochromes P450 and their associated reductases. Through these studies, we hope to ascertain whether this enzyme class represents a primary basis for the etiology or treatment of these neurodegenerative diseases.
Recent Publications
A model for the regulation of T-type Ca(2+) channels in proliferation: roles in stem cells and cancer. Gray LS, Schiff D, Macdonald TL. Expert Rev Anticancer Ther. 13:589-95 (2013).
Cost-effective and Large-scale synthesis of 16:0 Lysophosphatidic Acid. East JE, Macdonald TL. Synth Commun. 42:3614-3618 (2012).
Amixicile, a novel inhibitor of pyruvate: ferredoxin oxidoreductase, shows efficacy against Clostridium difficile in a mouse infection model. Warren CA, van Opstal E, Ballard TE, Kennedy A, Wang X, Riggins M, Olekhnovich I, Warthan M, Kolling GL, Guerrant RL, Macdonald TL, Hoffman PS. Antimicrob Agents Chemother. 56:4103-11 (2012).
Development of a phosphatase-resistant, L-tyrosine derived LPA1/LPA3 dual antagonist. East JE, Carter KM, Kennedy PC, Schulte NA, Toews ML, Lynch KR, Macdonald TL. Medchemcomm. 2:325-330 (2011).
Sphingosine kinase type 1 inhibition reveals rapid turnover of circulating sphingosine 1-phosphate. Kharel Y, Mathews TP, Gellett AM, Tomsig JL, Kennedy PC, Moyer ML, Macdonald TL, Lynch KR. Biochem J. 440:345-53 (2011).
James A. Marshall
In our research we identify natural products with interesting, often novel, structures and useful biological properties that may lead to medicinal agents for treatment of cancer, cardiovascular disorders and infectious diseases. We focus on key structural and stereochemical features present in these target natural products and devise mechanism or analogy based methodology for efficient construction of potentially useful subunits. Finally, we assemble the foregoing subunits by a combination of new and known reactions to synthesize the target substance and/or analogues thereof. Our overall program is thus target directed but methods driven.
Recent Publications
A cascade cyclization route to adjacent bistetrahydrofurans from chiral triepoxyfarnesyl bromides. Marshall JA, Hann RK. J Org Chem. 73, 6753-7(2008).
A formal synthesis of the callipeltoside aglycone. Marshall JA, Eidam PM. Org Lett. 10, 93-6 (2008).
Chiral allylic and allenic metal reagents for organic synthesis. Marshall JA. J Org Chem. 72, 8153-66 (2007).
ABC synthesis and antitumor activity of a series of Annonaceous acetogenin analogs with a threo, trans, threo, trans, threo-bis-tetrahydrofuran core unit. Marshall JA, Sabatini JJ, Valeriote F. Bioorg Med Chem Lett. 17, 2434-7 (2007).
Palladium- and copper-catalyzed 1,4-additions of organozinc compounds to conjugated aldehydes. Marshall JA, Herold M, Eidam HS, Eidam P. Org Lett. 8, 5505-8 (2006).
Synthesis of a bistetrahydrofuran C17-C32 fragment of the polyether antibiotic ionomycin. Marshall JA, Mikowski AM. Org Lett. 8, 4375-8 (2006).
Glenn J. McGarvey
Molecular Glycosciences
-
Complex Glycoconjugate Synthesis
-
Carbohydrate-Based Molecular Recognition
Cell surface carbohydrates, typically conjugated to proteins and lipids, are key elements in the cellular communication that is essential to cellular organization and function in all organisms. Not only do these cell surface constituents mediate normal behavior in cells, but they may also participate in molecular recognition events that are associated with many serious human diseases, including rheumatoid arthritis, viral and bacterial infections, and cancer metastasis. This provides an extremely attractive opportunity for selective chemical intervention of cell function through the targeting of specific cell surface carbohydrate structures.
A central theme of our research is the application of synthetic organic chemistry to the preparation of appropriate carbohydrate structures in order to carry out detailed structural studies addressing their recognition properties. The highly complex structures of carbohydrates, particularly in the form of their corresponding glycoconjugates, often challenge the limits of existing synthetic technology and dictate the development of new methodology. As such, studies addressing biological carbohydrates offer opportunities for discoveries in the both the chemical and biological domains.
One for the focuses of our efforts is the development of synthetic cancer vaccines directed toward cell surface carbohydrate antigen structures. In this context, the synthesis of complex oligosaccharides is currently under investigation, as well as the development of new strategies for the assembly of unnatural glycopeptide structures that may prove useful in the assembly of effective vaccines.
In other studies, we are endeavoring to exploit the dense stereochemical and functional content of carbohydrates to generate new classes of molecules for highly selective biorecognition. In particular, C-glycoside oligosaccharide/peptide hybrids are being examined for specific cell surface and DNA recognition and binding toward the goal of making available new methods for the treatment of diseases and for the rational modification of biological function.
 Representative Publications
Representative Publications
Studies on the stereoselective synthesis of C-allyl glycosides. McGarvey GJ, LeClair CA, Schmidtmann BA. Org Lett. 10, 4727-30 (2008).
The preparation of C-glycosyl amino acids – an examination of olefin cross-metathesis. Schmidtmann FW, Benedum TE, McGarvey GJ. Tetrahedron letters. 46, 4677-4681 (2005).
Synthesis of alpha- and beta-C-glycosides of N-acetylglucosamine. McGarvey GJ, Schmidtmann FW, Benedum TE, Keizer DE. Tetrahedron letters. 44, 3775-3779 (2003).
Development of co- and post-translational synthetic strategies to C-neoglycopeptides. McGarvey GJ, Benedum TE, Schmidtmann FW. Org Lett. 4, 3591-4 (2002).
Carl Trindle
Research in Computational Modeling and Theoretical Chemistry
In the past twenty-five years molecular mechanics and quantum mechanical methods have become essential helpers to experimental chemists of all kinds. Modern software systems teamed with powerful computers now make possible practical representation of many aspects of chemical structure and reactivity. I help newcomers to modeling to develop good judgment in their use of these techniques, and apply some of the most powerful techniques to chemical problems.
I use computer modeling to study reaction pathways in organic systems, structures and energetics of systems likely to possess low-lying states of high spin, and the bonding and reactivity of metal-organic complexes. Much of my work is done in collaboration with graduate students conducting experimental investigations with other UVA faculty.
Some projects I am pursuing now:
- Modeling of aryl carbenes, so to characterize Tomioka’s long-lived methylene species (Presented at the International Symposium on Reactive Intermediates and Unusual Molecules [ISRIUM], Nara Japan, Sept 2001.)
- Characterization of Dearomatization induced by complexation with Osmium, Rhenium, and Tungsten species (presented at the Faraday Discussion York University, April 2003)
- Stereochemical consequences of weak interactions loosely termed “hydrogen bonding” (presented in preliminary form at Rutgers University, February, 2004)
- Characterization of Closed shell and open shell dianions stable with respect to autoionization and dissociation (Presented at ISRIUM, Edinburgh UK August 2005)
- TDDFT characterization of circular dichroism spectra of high symmetry organic species (presented at the ACS National Meeting in New Orleans, April 2008
- Structural characterization of organic cations which bind protons and the resulting dications (to be presented at the Int Conf of Quantum Chemistry, Helsinki June 2009
- TDDFT characterization of fluorescence spectra of Iridium and Ruthenium systems

Computed anharmonic frequencies for Formic Acid Dimers can guide experimental detection of short-lived isomers [figure by Ilhan Yavuz].
Recent Publications
Environmental Sensitivity of Ru (II) Complexes: The Role of the Accessory Ligands, Eileen N. Dixon †, Michael Z. Snow †, Jennifer L. Bon †, Alison M. Whitehurst †, Benjamin A. DeGraff *†, Carl Trindle ‡, and James N. Demas ‡, Inorg. Chem., 2012, 51 (6), pp 3355–3365
Communication: Frequency shifts of an intramolecular hydrogen bond as a measure of intermolecular hydrogen bond strengths, Q Gu, C Trindle, JL Knee, J. Chem. Phys. 2012 137, 091101
Structure and energetics of cyclopropane carboxaldehyde. Trindle, C., Bleda, E. A. and Altun, Z. (2012), . Int. J. Quantum Chem.. doi: 10.1002/qua.24201
Computational thermochemistry of glycolaldehyde. Bleda, E. A., Yavuz, I., Altun, Z. and Trindle, C. (2012), . Int. J. Quantum Chem.. doi: 10.1002/qua.24200
Photophysical and Analyte Sensing Properties of Cyclometalated Ir (III) Complexes, Leavens BB, Trindle CO, Sabat M, Altun Z, Demas JN, DeGraff BA., J Fluoresc. 2012 , 22:163-74


