
W. Dean Harman
Education
B.S. Stanford University, 1983
Ph.D. Stanford University, 1987
Research Associate, Stanford University, 1988-89
For decades, the dearomatization of arenes has been recognized as a chemical transformation of fundamental importance. It provides the connection between this robust and abundant source of hydrocarbons and the alicyclic frameworks common to many biologically active products. Thus, dearomatization methods have become powerful tools for organic synthesis.
The chemical properties of arenes are influenced by their coordination to a transition metal. For example, complexes such as (η6-arene)Cr(CO)3+ and its cationic analogs (e.g., FeCp+, RuCp+, Mn(CO)3+) are susceptible to nucleophilic substitution or addition, ultimately leading to the formation of substituted arenes or cyclohexadienes, respectively. During the past three decades, the application of η6-arene complexes to organic synthesis has been widely explored.
In the Harman Research Group, an alternative approach to the dearomatization of arenes has been developed in which the aromatic system may be activated toward organic transformations by η2-coordination. Here, the metal-arene bond is stabilized primarily by the interaction of filled metal dπorbitals with the π* system of the arene, an interaction having two important consequences for the activation of the aromatic ring. Through πbackbonding, the aromatic π system becomes more electron-rich, similar to what is observed for organic arenes bearing electron-donating groups. Additionally, structural data for complexes containing η2-coordinated aromatic rings show significant distortions in the bond lengths of the ring consistent with a localization of πelectron density. Together, these effects activate η2-bound aromatic systems toward electrophilic rather than nucleophilic addition. When the π base binds an aromatic heterocycle such as a pyridine, furan or pyrrole, novel tandem addition reactions and cycloaddition reactions become possible.
Of the handful of transition metal systems that are known to form stable η2 complexes with aromatic molecules, only d6 octahedral metal complexes have been shown to enhance the reactivity of the aromatic ligand toward electrophiles to date. For nearly a decade, despite our best efforts, this mode of arene activation was known only for the pentaammineosmium(II) system. However, in the past few years a new generation of dearomatization agents have been developed in our group based on a careful matching of the d5/d6 reduction potential of rhenium(I), tungsten(0), and molybdenum(0) complexes with that of pentaammineosmium(II).
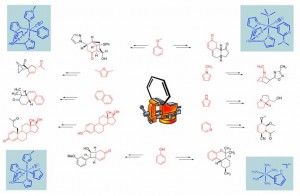
Recent Publications
Group 6 Dihapto-Coordinate Dearomatization Agents for Organic Synthesis Liebov, B. K.; Harman, W. D., Chem. Rev. 2017, 117, 13721-13755.
Molybdenum-Promoted Synthesis of Isoquinuclidines with Bridgehead CF3 Groups Wilde, J. H.; Smith, J. A.; Dickie, D. A.; Harman, W. D., J. Am. Chem. Soc. 2019, 141, 18890-18899.
Highly Functionalized Cyclohexenes Derived from Benzene: Sequential Tandem Addition Reactions Promoted by Tungsten Wilson, K. B.; Smith, J. A.; Nedzbala, H. S.; Pert, E. K.; Dakermanji, S. J.; Dickie, D. A.; Harman, W. D., J. Org. Chem. 2019, 84, 6094-6116.
Preparation of Cyclohexene Isotopologues and Stereoisotopomers from Benzene Smith, J. A.; Wilson, K. B.; Sonstrom, R. E.; Kelleher, P. J.; Welch, K. D.; Pert, E. K.; Westendorff, K. S.; Dickie, D. A.; Wang, X.; Pate, B. H.; Harman, W. D., Nature 2020, 581, 288-293.
Experiments and Direct Dynamics Simulations that Probe η2-Arene/Aryl-Hydride Equilibria of Tungsten Benzene Complexes Smith, J. A.; Schouten, A.; Wilde, J. H.; Westendorff, K. S.; Dickie, D. A.; Ess, D. H.; Harman, W. D., Journal of the American Chemical Society 2020, 142, 38, 16437–16454.
