Lin Pu
Education
Ph.D. University of California San Diego, 1990
Postdoctoral Fellow, Stanford University, 1991-1992
Postdoctoral Fellow, California Institute of Technology, 1992-1994
Organic, Polymer and Organometallic Chemistry; Asymmetric Catalysis; Chiral Sensors; Optically Active Materials
Multi-disciplinary research programs involving organic synthesis, molecular recognition, fluorescent sensing, asymmetric catalysis, and polymers are conducted in our laboratory. The 1,1′-bi-2-naphthol (BINOL) and its derivatives are chosen as the chiral building blocks to construct novel chiral molecules and macromolecules for diverse applications. We have developed a family of enantioselective fluorescent sensors for the recognition of organic molecules such as alpha-hydroxycarboxylic acids, amino acids, amino alcohols, and amines. These sensors are potentially useful for rapid assay of the enantiomeric composition of chiral compounds and for high throughput chiral catalyst screening. They are also potentially useful for biological analysis and imaging. New chiral conjugated polymers and dendrimers are prepared for applications in materials, catalysis, and sensing. We have discovered that the Lewis acid complexes of the optically active binaphthyl molecules and polymers can carry out highly enantioselective organic reactions such as organozinc additions to aldehydes, hetero-Diels-Alder reactions, 1,3-dipolar cycloadditions, reductions of ketones, Michael additions, epoxidations, and others. Interesting chiral organic molecules including those of biological functions are prepared by using these catalysts.
Enantioselective Fluorescent Sensors
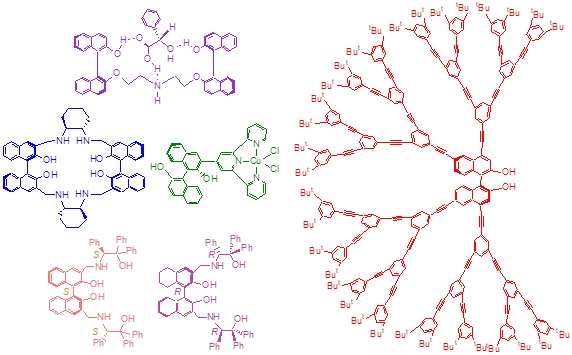
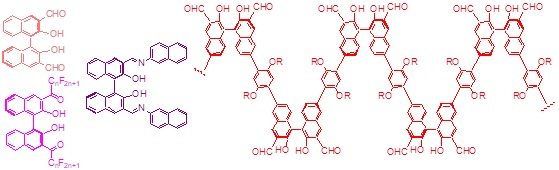
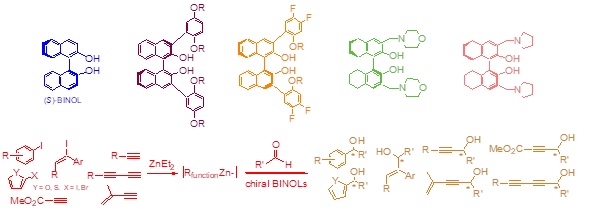
Asymmetric Catalysts
Recent Publications
Regioselective Substitution of BINOL. Pu, L. Chem. Rev. 2024, 124, 6643−6689.
Chemoselective and Enantioselective Fluorescent Identification of Specific Amino Acid Enantiomers. Pu, L. A Feature Article in Chem. Comm. 2022, 58, 8038-8048.
Enantioselective Fluorescent Recognition of Free Amino Acids: Challenges and Opportunities. Pu, L. Angew. Chem. Int. Ed. 2020, 59, 21814-21828.
Free Amino Acid Recognition: A Bisbinaphthyl-Based Fluorescent Probe with High Enantioselectivity. Zhu, Y.-Y.; Wu, X.-D.; Gu, S.-X.; Pu, L. J. Am. Chem. Soc. 2019, 141, 175−181.
Simultaneous Determination of Concentration and Enantiomeric Composition in Fluorescent Sensing. Lin Pu. Acc. Chem. Res. 2017, 50, 1032-1040.
Regiospecific Hydration of N-(Diphenylphosphinoyl)propargyl amines: Synthesis of b-Amino Ketones by Au(III) Catalysis. Ying, J.; Pu, L. J. Org. Chem. 2016, 81, 8135−8141. A Featured Article. Highlight on the cover.
Conjugated polymer-enhanced enantioselectivity in fluorescent sensing. Zhang, X. –P.; Wang, C.; Wang, P.; Du, J. –J.; Zhang, G. –Q.; Pu, L. Chem. Sci. 2016, 7, 3614–3620.
Rational Design of a Fluorescent Sensor to Simultaneously Determine Both the Enantiomeric Composition and Concentration of Chiral Functional Amines. Wen, K. –L.; Yu, S. –S.; Huang, Z.; Chen, L. –M.; Xiao, M.; Yu, X. –Q.; Pu, L. J. Am. Chem. Soc. 2015, 137, 4517-4524.
Asymmetric Functional Organozinc Additions to Aldehydes Catalyzed by BINOLs. Pu, L. Acc. Chem. Res. 2014, 47, 1523–1535.
Zn(II) Promoted Dramatic Enhancement in the Enantioselective Fluorescent Recognition of Chiral Amines by a Chiral Aldehyde. Huang, Z.; Yu, S. S.; Yu, X. Q.; Pu. L. Chem. Sci. 2014, 5, 3457-3462.
Enantioselective Fluorescent Sensors: A Tale of BINOL. Pu, L. Acc. Chem. Res. 2012, 45, 150–163.
Simultaneous Determination of Both the Enantiomeric Composition and Concentration of a Chiral Substrate with One Fluorescent Sensor. Yu, S.; Plunkett, W.; Kim, M.; Pu, L. J. Am. Chem. Soc. 2012, 134, 20282–20285. A spotlight article reported in J. Am. Chem. Soc., 2013, 135 (3), 949–950.
1,1'-BINAPHTHYL-BASED CHIRAL MATERIALS: OUR JOURNEY

