- People
- Research
- Graduate
- Undergraduate
- Prospective & Transfer Students
- General Chemistry Options
- Policies
- Tutors
- Pre-Health
- Undergraduate Advisors
- Chemistry Major
- Process for Declaring a Major, Minor, DMP, or ACS Certification
- B.A. in Chemistry
- B.S. Chemistry
- B.S. Specialization in Biochemistry
- B.S. Specialization in Chemical Education
- B.S. Specialization in Chemical Physics
- B.S. Specialization in Environmental Chemistry
- B.S. Specialization in Materials Science
- B.A./M.S. or B.S./M.S. in Chemistry ("3+1" Degree Option)
- Undergraduate Research
- Distinguished Majors Program
- Minor
- Forms
- Study Abroad
- Undergraduate FAQs
- Undergraduate Resources
- Safety
- Seminars
- Newsletter
Muhammad Murtaza Hassan (Beginning August 2025)
Assistant Professor of Chemistry
Education
HBSc, Chemistry Specialist & Math Minor, University of Toronto Mississauga, 2013
MSc, Masters in Organic Chemistry, York University, 2017
PhD, Doctorate in Medicinal Chemistry, University of Toronto Mississauga, 2021
Postdoctoral Studies, Stanford University, 2021-2025
Research
Like drug design? We are building the blueprint for generalizable, rationally designed molecular glue drugs that could transform the future of targeted cancer therapy.
Image
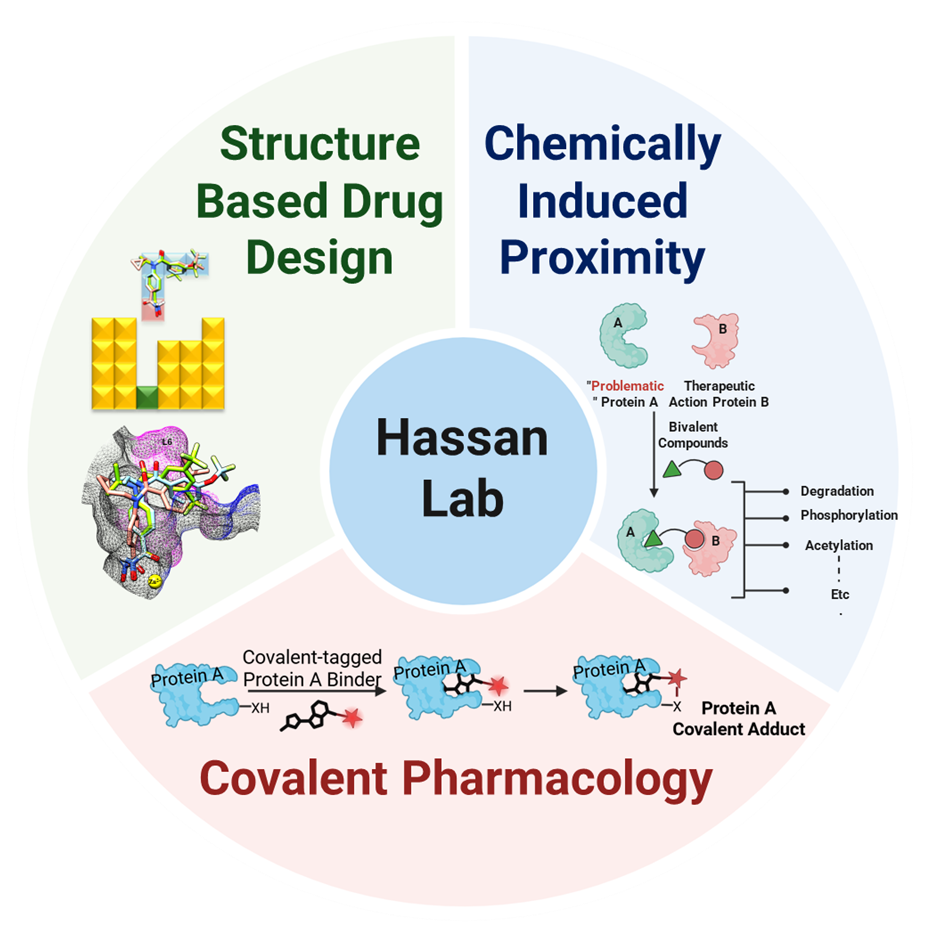
The Hassan Lab focuses on developing cutting-edge therapeutics at the interface of chemically induced proximity (CIP), covalent pharmacology, and structure-based rational drug design.
Proximity-driven interactions between biomolecules underpin nearly all cellular processes. By artificially harnessing this principle using synthetic molecules, chemically induced proximity (CIP) offers a transformative strategy that moves beyond traditional drug discovery — which typically focuses on inhibiting protein function — toward a new pharmacological paradigm: hijacking endogenous cellular machinery to induce targeted protein degradation, phosphorylation, acetylation, and more.
Advancements in structural biology, along with a deeper mechanistic understanding of cellular machinery, provide a powerful foundation for structure-based drug design. Leveraging shape complementarity and specific intermolecular interactions allows for the development of highly potent and selective drugs — capable of selectively targeting cells that express disease-driving proteins, such as oncoproteins in cancer.
When coupled with covalent chemistry, structure-based drug design gains additional advantages: increased enthalpic binding, longer duration of action, improved potency and selectivity, and reduced manufacturing costs. Covalent chemistry also expands the druggable proteome by offering reactive “hooks” that form bonds with nucleophilic residues in shallow or otherwise undruggable protein surfaces.
Our research centers on the rational integration of covalent chemistry into structure-guided CIP strategies. We believe this approach holds great promise for expanding the toolbox of targeted protein degradation and for enabling the drugging of “undruggable” targets.
Research Focus: A key challenge in translating protein degraders from bench to bedside is their typical reliance on bivalent design. While bivalency enables a modular, plug-and-play approach, the resulting compounds are often large — frequently exceeding traditional drug-likeness criteria such as molecular weight, number of rotatable centers, hydrogen bonding capacity etc. In contrast, monovalent degraders (or “molecular glues”) offer comparable pharmacological benefits with significantly improved drug-like properties. However, these are often discovered serendipitously, and a generalizable chemical design strategy has yet to emerge.
This bottleneck limits the pharmaceutical industry’s ability to advance molecular glue degraders into the clinic.
The Hassan Lab is addressing this gap by developing first-in-class, rationally designed monovalent degraders with drug-like properties that selectively target cancer cells and improve patient outcomes.
Interested in our work? Contact us at hassan36@stanford.edu.
What are we looking for?
At the Hassan Lab, we welcome trainees at all levels — including postdoctoral fellows, graduate students, and undergraduate researchers — who are excited to push the boundaries of drug discovery and chemical biology. We are looking for self-motivated, creative individuals who thrive in a collaborative, team-oriented environment. We value diversity in all its forms — of thought, background, and culture — and are committed to building a lab culture that is fun, inclusive, intellectually vibrant, and driven by curiosity and purpose. If you're passionate about science and want to grow in a space that nurtures both technical excellence and personal development, we’d love to hear from you.
What we offer?
The Hassan Lab provides a rigorous, high-energy research environment with access to state-of-the-art resources and global scientific collaborations. Trainees gain hands-on expertise in synthetic chemistry, biochemistry, molecular and cellular biology, supported by cutting-edge instrumentation including LC-MS, HPLC, and flash chromatography systems and more. Our research is highly translational, directly addressing clinically relevant problems in oncology and beyond, with strong potential for spinning out high-impact projects into biotech startups. We are embedded within the UVA Comprehensive Cancer Center and maintain active collaborations with world-class institutions and pioneers in the field, such as the Dana-Farber Cancer Institute (Harvard Medical School), Fred Hutchinson Cancer Center, and more. We’re committed to fostering scientific excellence while providing the mentorship, technical training, and collaborative opportunities needed for your success — whether in academia, biotech, or beyond.
Select Publications and Key Findings
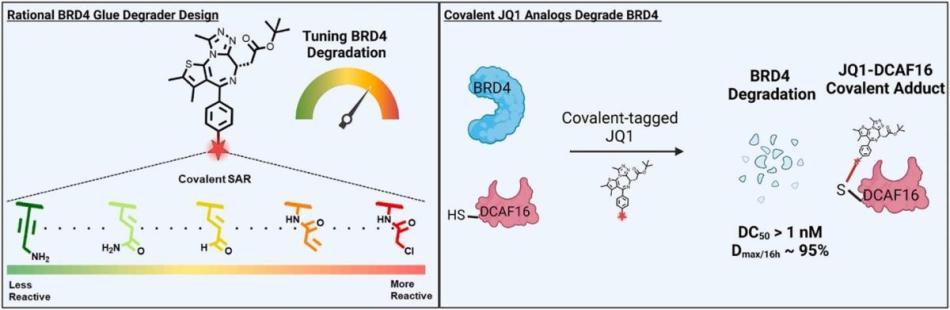
Hassan, M.M* et al., Exploration of the Tunability of BRD4 Degradation by DCAF16 Trans-labelling Covalent Glues, Eur. J. Med. Chem. 116904. 2024. doi:10.1016/j.ejmech.2024.116904
Image
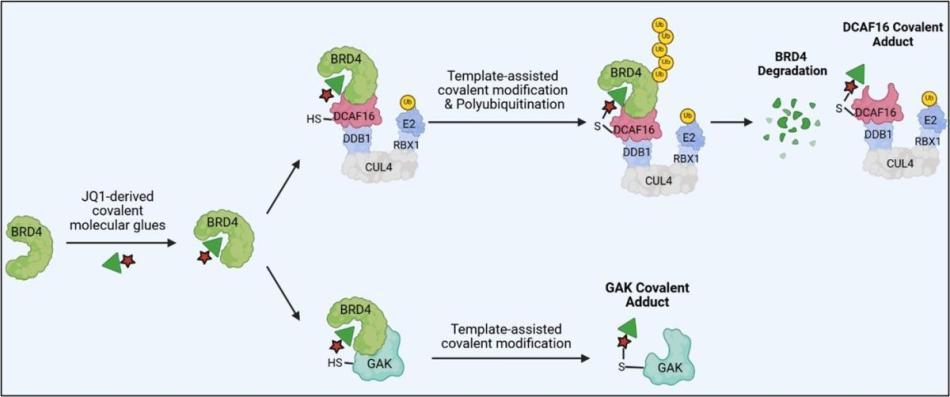
Li, Y.D.*, Ma, M.*, Hassan, M.M.* et al., Template-assisted covalent modification of DCAF16 enables BRD4 molecular glue degraders, Nat. Chem. Biol. 2024. DOI: 10.1038/s41589-024-01668-4
Image
Hassan, M.M.* et al., Characterization of Conformationally Constrained Benzanilide scaffolds for Potent and Selective HDAC8 targeting, J. Med. Chem., 2020, 63 (15), 8634–8648
Image
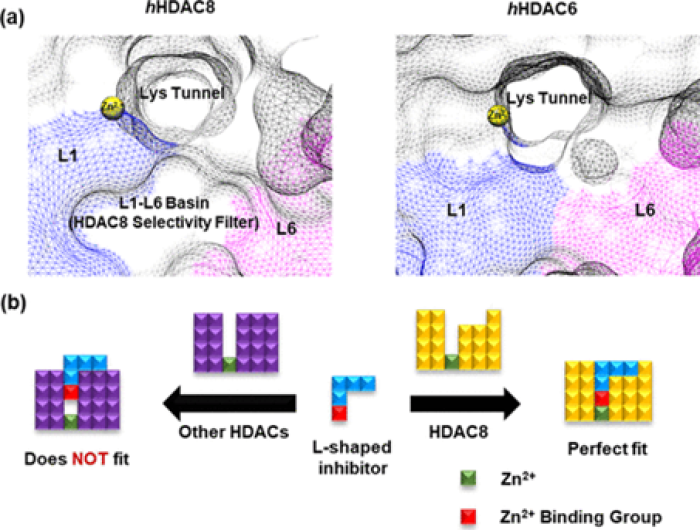
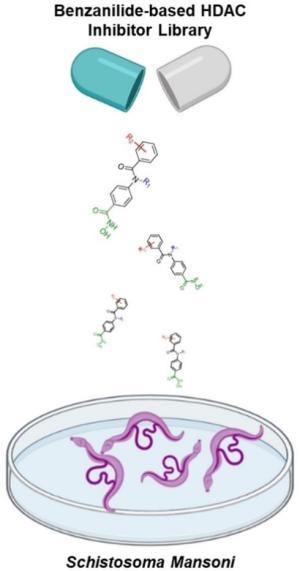
Hassan, M.M.* et al., Phenotypic Screening of Histone deacetylase (HDAC) Inhibitors against Schistosoma Mansoni, ChemMedChem., 2022, 17, (18), e202100622.
Image