John H. Bushweller
Education
B.A. Dartmouth College, 1984
Ph.D. University of California, Berkeley, 1989
NIH Postdoctoral Fellow, ETH-Zurich, Switzerland,1990-1992
Structure/Function Studies of Transcription Factor Drivers in Cancer
Our lab is fundamentally interested in understanding how transcription factors that are drivers in cancer mediate their effects. This basic science understanding is essential to develop new approaches to cancer treatment. Our approach to do this is based on structure/function studies. We determine 3D structures of functional domains from these transcription factors that mediate specific protein-protein or protein-nucleic acid interactions bound to their interaction partner. Based on the structural data, we develop specific point mutations which disrupt this specific interaction but do not affect the structure or stability of the domain. These mutant forms of the transcription factor serve as high quality biological reagents to carry out functional studies with. Furthermore, they recapitulate what a small molecule inhibitor of the interaction would do, so they serve as tools to validate specific interactions for inhibitor development. Using these well-validated biological tools, we probe functional effects including, but not limited to, effects on proliferation, effects on differentiation (flow cytometry), effects on gene expression (RNA-Seq), effects on transcription factor occupancy (ChIP-Seq), effects on epigenetic signaling (ChIP-Seq), and effects in relevant mouse models of the cancer (latter is done with a group of outstanding collaborators at other institutions).
A long-term focus has been structural studies of a novel transcription factor referred to as the core-binding factor (CBF) which is a heterodimeric transcription factor (CBFβ and RUNX1, 2, or 3). CBFβ/RUNX1 is essential for hematopoietic development. Gene translocations associated with the genes coding for CBFβ and RUNX1 produce novel fusion proteins (CBFβ-SMMHC, RUNX1-ETO, TEL-RUNX1) which have been implicated as playing a role in more than 30% of acute leukemias. We have carried out extensive structural and functional studies of the fusion protein forms of CBFβ and RUNX1. We have extended these studies to the MLL protein, a key epigenetic regulator that is the target of chromosomal translocations (MLL-AF9, MLL-ENL, MLL-AF4, etc.) in leukemia which are particularly poor prognosis.
Drug Development Targeting Transcription Drivers in Cancer
Dysregulation of gene expression is a hallmark of all cancers. It is critical for self-renewal and chemo-resistance of cancer cells, which contribute to the inability to completely eradicate cancer cells, thereby leading to relapse. The specific gene expression program that confers these properties derives from the aberrant activity of specific transcription factors that are drivers of disease. Clearly, the most direct and effective approach to alter this gene expression program is to directly target the activity of the transcription factors driving the disease. There are numerous examples of such transcription factor drivers in cancer such as fusion proteins of RUNX1 and CBFβ in leukemia, fusion proteins of ERG in prostate cancer and Ewing’s sarcoma, ETV-1 in melanoma, other members of the Ets family of transcription factors in a variety of different cancers, etc. Transcription factors were traditionally viewed as “undruggable” due to the need to target more challenging protein-protein or protein nucleic acid interactions through which these proteins act. There are still relatively few examples of such agents for cancer treatment, with the MDM2-p53 inhibitors being one example of such an agent that has progressed to the clinic. We are exploring several unique approaches to target this important class of proteins for drug development.
We have developed an inhibitor targeting the CBFβ-SMMHC fusion protein that occurs in inv(16) acute myeloid leukemia (AML). This inhibitor is a protein-protein interaction inhibitor that restores RUNX1 function in these cells. We have shown it is effective in a mouse model of inv(16) AML as well as against human inv(16) AML patient cells. Furthermore, we have shown that much of the effect of the compound is mediated by a dramatic reduction in expression of MYC, a key transcription factor driver in many cancers. This drug has been licensed to Systems Oncology and is progressing toward clinical testing.
Recent Publications
Pulikkan JA, Hegde M, Belaghzal H, Illendula A, Yu J, Ahmed H, O’Hagan K, Ou J, Muller-Tidow C, Wolfe SA, Zhu LJ, Dekker J, Bushweller JH, Castilla LH. CBFβ-SMMHC inhibition leads to alteration of chromatin dynamics at MYC distal enhancers and abrogation of inv(16) leukemia. Cell. June 28, 2018 174(1):172-186. PubMed PMID: 29958106.
Illendula A, Pulikkan JA, Zong H, Grembecka J, Xue L, Sen S, Zhou Y, Boulton A, Kuntimaddi A, Gao Y, Rajewski RA, Guzman ML, Castilla LH, Bushweller JH. Chemical biology. A small-molecule inhibitor of the aberrant transcription factor CBFβ-SMMHC delays leukemia in mice. Science. 2015 Feb 13;347(6223):779-84. PubMed PMID: 25678665
Illendula A, Gilmour J, Grembecka J, Tirumala VSS, Boulton A, Kuntimaddi A, Schmidt C, , Wang L, John A. Pulikkan, Hongliang Zong, Mahmut Parlak, Cem Kuscu, Anna Pickin, Yunpeng Zhou, Yan Gao Y, Mishra L, Adli M, Castilla LH, Rajewski RA, Janes KA, Guzman ML, Bonifer C, and Bushweller JH. Small Molecule Inhibitor of CBFb-RUNX Binding for RUNX Transcription Factor Driven Cancers. EBioMedicine. 2016 8: 117-131. Pubmed PMID: 27428424
Kuntimaddi A, Achille NJ, Thorpe J, Lokken AA, Singh R, Hemenway CS, Adli M, Zeleznik-Le NJ, Bushweller JH. Degree of recruitment of DOT1L to MLL-AF9 defines level of H3K79 Di- and tri-methylation on target genes and transformation potential. Cell Rep. 2015 May 5;11(5):808-20. PubMed PMID: 25921540
Cierpicki T, Risner LE, Grembecka J, Lukasik SM, Popovic R, Omonkowska M, Shultis DD, Zeleznik-Le NJ, Bushweller JH. Structure of the MLL CXXC domain-DNA complex and its functional role in MLL-AF9 leukemia. Nat Struct Mol Biol. 2010 Jan;17(1):62-8. PubMed PMID: 20010842
Awards and Honors
- Dr. Bushweller named 2020 Harrison Distinguished Teaching Professor
- Dr. Bushweller receives grant from Alex’s Lemonade Stand
- A small-molecule inhibitor of the aberrant transcription factor CBFβ-SMMHC delays leukemia in mice, featured in Science
- NIH Independent Scientist Award, 1998-2002
- Dr. Bushweller receives grant from Falk Foundation and Leukemia & Lymphoma Society for development of AML1-ETO inhibitors
David S. Cafiso
Education
A.B. University of California, Berkeley, 1974
Ph.D. University of California, Berkeley, 1979
Postdoctoral Fellow, Stanford University, 1980
Membrane Proteins and Cell Signaling
Membranes and membrane proteins participate in some of the most important and interesting cellular processes. Energy transduction, cell signaling, membrane excitability, secretion and immune recognition are examples of a few of the processes mediated by membrane proteins. However, the molecular mechanisms by which lipids and membrane proteins accomplish these tasks are largely unknown. We primarily use EPR spectroscopy and high-resolution NMR to investigate the structure and function of membrane proteins.
Protein – membrane surface interactions
Attachment is critical for cell-signaling because it controls protein-protein interactions and the access of enzymes to lipid substrates. We are currently determining the structure and electrostatic interactions made by highly positively charged protein motifs, such as those from MARCKS (the myristoylated alanine rich C-kinase substrate) with negatively charged lipid surfaces. In addition to regulating membrane attachment, these positively charged motifs function to sequester phosphatidylinositol 4,5, bisphosphate (PIP2), and regulate the activity of this phosphorylated inositol lipid within the cytoplasmic membrane. We are also determining the membrane interactions made by protein domains such as C2 domains. C2 domains perform critical roles in membrane trafficking, membrane fusion and membrane repair, and defects in these domains result in forms of muscular dystrophy and deafness.
Active transport across membranes
We are determining the molecular mechanisms by which BtuB transports vitamin B12 across the outer membrane of Escherichia coli. This protein is homologous to FecA, FepA and FhuA, outer membrane iron transport proteins that presumably function by similar mechanisms. These proteins belong to a class of transport proteins for which high-resolution structural models have been obtained, and they are extremely important for the survival of some bacterial pathogens.
Recent Publications
Allosteric control of syntaxin 1a by Munc18-1: characterization of the open and closed conformations of syntaxin. Dawidowski D, Cafiso DS. Biophys J. 104:1585-94 (2013).
Monomeric TonB and the Ton box are required for the formation of a high-affinity transporter-TonB complex. Freed DM, Lukasik SM, Sikora A, Mokdad A, Cafiso DS. Biochemistry. 52:2638-48 (2013).
Taking the pulse of protein interactions by EPR spectroscopy. Cafiso DS. Biophys J. 103:2047-8 (2012).
Ligand-induced structural changes in the Escherichia coli ferric citrate transporter reveal modes for regulating protein-protein interactions. Mokdad A, Herrick DZ, Kahn AK, Andrews E, Kim M, Cafiso DS. J Mol Biol. 423:818-30 (2012).
Solution structure of the ESCRT-I and -II supercomplex: implications for membrane budding and scission. Boura E, Różycki B, Chung HS, Herrick DZ, Canagarajah B, Cafiso DS, Eaton WA, Hummer G, Hurley JH. Structure. 20:874-86 (2012).

Sergei A. Egorov
Education
B.Sc. Leningrad University, 1987
Ph.D. University of Wisconsin, 1996
Postdoctoral Research Associate, Columbia University,1996-1997
Structure and Dynamics in Supercritical Fluids
Supercritical fluids (SCFs) are currently receiving much industrial and scientific interest as a result of their unique physical properties. The most characteristic features of SCFs are liquid-like densities, gas-like viscosities, and diffusivities that are intermediate between typical gas and liquid values. The resulting combination of high dissolving power and enhanced mass-transfer rates makes SCFs attractive alternatives to liquid solvents for a variety of industrial applications, such as extraction, separation and reaction processes. In addition, the high compressibility of SCFs in the near-critical region allows one to tune their properties to desired values by applying small changes in pressure, which in turn makes it possible to tailor the rates and selectivities of chemical processes. Since the aforementioned applications of SCFs generally involve dilute solutions, it is essential to develop a microscopic understanding of the structure and dynamics of a supercritical solvent in the vicinity of a solute.
We use the methods of classical statistical mechanics, such as integral equation theory and mode coupling theory, to study structural and dynamical properties of supercritical solutions. Some of the problems we address are as follows. How does the solvent-solute and solute-solute clustering affect the rates and equilibrium constants of chemical reactions in SCFs? How does the proximity to the critical point affect the transport properties and what are the ramifications for diffusion controlled chemical reactions? How is preferential solvation manifested in local composition effects in dilute supercritical solutions? The answers to these questions should help us shed further light on fundamental properties of SCFs and their practical applications.
Quantum and Semiclassical Many-Body Dynamics
Numerous problems in chemical physics involve calculations of quantum time correlation functions (TCFs) in many-body systems. Particular examples include: medium-induced electron transfer, dissipative tunneling, radiationless processes, and electronic spectroscopy of chromophores in crystals and in liquids. While certain systems require a fully quantum mechanical treatment, there exists a large class of systems of chemical interest for which classical mechanics provides a reasonably good approximation. An appealing approach to the calculation of TCFs for such systems involves using semiclassical methods, which are generally based on the assumption that quantum effects can be taken into account by introducing relatively small corrections to the classical results. However, the shorter the time scale on which the behavior is analyzed, the more important quantum corrections may become, even for systems which are classical as far as their static and low-frequency dynamical properties are concerned. One of the research projects in our group involves developing a systematic procedure for including quantum effects into the results for TCFs obtained from classical simulations.
An alternative approach to study quantum dynamics in condensed phases involves calculating imaginary-time correlation functions using path integral Monte Carlo (PIMC) method and performing analytic continuation to the real-time axis. Unfortunately, analytic continuation is numerically unstable, and therefore leads to uncontrollable amplification of statistical noise unavoidable in PIMC simulations. In our group we employ the information theory and the methodology from the field of inverse problems in order to develop various techniques, such as Maximum Entropy and Singular Value Decomposition, for stabilizing the procedure of analytic continuation of quantum imaginary-time TCFs.
Recent Publications
S. N. Merz, Z. J. Farrell, J. Pearring, E. Hoover, M. Kester, S. A. Egorov, D. L. Green, and K. H. DuBay, "Computational and Experimental Investigation of Janus-like Monolayers on Ultrasmall Noble Metal Nanoparticles", ACS Nano, 12, p. 11031-11040, (2018).
A. Milchev, S. A. Egorov, A. Nikoubashman, and K. Binder, "Nematic order in solutions of semiflexible polymers: Hairpins, elastic constants, and the nematic-smectic transition", J. Chem. Phys., 149, p. 174909, (2018).
K. E. Klop, R. P. A. Dullens, M. P. Lettinga, S. A. Egorov, and D. G. A. L. Aarts, "Capillary nematisation of colloidal rods in connement", Mol. Phys., 116, p. 2864-2871, (2018).
R. J. Chen, R. Poling-Skutvik, M. P. Howard, A. Nikoubashman, S. A. Egorov, J. C. Conrad, and J. C. Palmer, "Influence of polymer flexibility on nanoparticle dynamics in semidilute solutions", Soft Matter, 15, p. 1260-1268, (2019).
S. J. Wang, D. Venkateshvaran, M. R. Mahani, U. Chopra, E. R. Mc-Nellis, R. Di Pietro, S. Schott, A. Wittmann, G. Schweicher, M. Cubukcu, K. Kang, R. Carey, T. J. Wagner, J. N. M. Siebrecht, D. P. G. H. Wong, I. E. Jacobs, R. O. Aboljadayel, A. Ionescus, S. A. Egorov, S. Mueller, O. Zadvorna, P. Skalski, C. Jellett, M. Little, A. Marks, I. McCulloch, J. Wunderlich, J. Sinova, H. Sirringhaus, "Long spin diffusion lengths in doped conjugated polymers due to enhanced exchange coupling", Nature Electronics, 2, p. 98-107, (2019).
J. Midya, Y. Cang, S. A. Egorov, K. Matyjaszewski, M. R. Bockstaller, A. Nikoubashman, and G. Fytas, "Disentangling the Role of Chain Conformation on the Mechanics of Polymer Tethered Particle Materials", Nano Letters, 19, p. 2715-2722, (2019).
S. N. Merz, E. Hoover, S. A. Egorov, K. H. DuBay, and D. L. Green, "Predicting the effect of chain-length mismatch on phase separation in noble metal nanoparticle monolayers with chemically mismatched ligands", Soft Matter, 15, p. 4498-4507 (2019).
J. Midya, S. A. Egorov, K. Binder, and A. Nikoubashman, "Phase behavior of exible and semi exible polymers in solvents of varying quality", J. Chem. Phys., 151, p. 034902 (2019).
U. Chopra, S. A. Egorov, J. Sinova, and E. R. McNellis, "Chemical and structural trends in the spin-admixture parameter of organic semiconductor molecules", J. Phys. Chem. C, 123, p. 19112-19118 (2019).
U. Chopra, S. Shambhawi, S. A. Egorov, J. Sinova, and E. R. McNellis, "Accurate and general formalism for spin-mixing parameter calculations", Phys. Rev. B, 100, p. 134410 (2019).

Linda Columbus
Education
B.A. Smith College, 1996
Ph.D. University of California, Los Angeles, 2001
NIH Postdoctoral Fellow, The Scripps Research Institute, 2002 - 2007
Membrane proteins facilitate the transfer of information across lipid bilayers, comprise approximately 25% of a typical proteome, and represent over half of all drug targets. The membrane proteins that mediate interactions between bacterial pathogens and hosts are of particular interest to our laboratory. Invasive bacterial pathogens are responsible for many lethal diseases and epidemics, including plague and meningitis. Although these bacteria have diverse mechanisms of cellular invasion, all of the pathways rely upon interactions between host and bacterial membrane proteins.
Our lab seeks to determine the structure and conformational changes of membrane proteins involved in bacterial infection using a combination of site-directed spin labeling (SDSL), nuclear magnetic resonance (NMR) spectroscopy, and X-ray crystallography, and also to develop tools to accelerate membrane protein structure determination by these methods.
Recent Publications
Refinement of Highly Flexible Protein Structures using Simulation-Guided Spectroscopy. Hays JM, Kieber MK, Li JZ, Han JI, Columbus L, Kasson PM. Angew Chem Int Ed Engl. 2018 Dec 21;57(52):17110-17114. doi: 10.1002/anie.201810462. Epub 2018 Nov 27. PMID: 30395378.
Low- q Bicelles Are Mixed Micelles. Caldwell TA, Baoukina S, Brock AT, Oliver RC, Root KT, Krueger JK, Glover KJ, Tieleman DP, Columbus L. J Phys Chem Lett. 2018 Aug 2;9(15):4469-4473. doi: 10.1021/acs.jpclett.8b02079. Epub 2018 Jul 25. PMID: 30024762.
Conformation transitions of the polypeptide-binding pocket support an active substrate release from Hsp70s. Yang J, Zong Y, Su J, Li H, Zhu H, Columbus L, Zhou L, Liu Q. Conformation transitions of the polypeptide-binding pocket support an active substrate release from Hsp70s. Nat Commun. 2017 Oct 31;8(1):1201. doi: 10.1038/s41467-017-01310-z. PubMed PMID: 29084938; PubMed Central PMCID: PMC5662698.
Modulating Vascular Hemodynamics With an Alpha Globin Mimetic Peptide (HbαX).Keller TC 4th, Butcher JT, Broseghini-Filho GB, Marziano C, DeLalio LJ, Rogers S, Ning B, Martin JN, Chechova S, Cabot M, Shu X, Best AK, Good ME, Simão Padilha A, Purdy M, Yeager M, Peirce SM, Hu S, Doctor A, Barrett E, Le TH, Columbus L, Isakson BE. Hypertension. 2016 Dec;68(6):1494-1503. Epub 2016 Oct 31.
Opa+ Neisseria gonorrhoeae has reduced survival in human neutrophils via Src family kinase-mediated bacterial trafficking into mature phagolysosomes. Johnson MB, Ball LM, Daily KP, Martin JN, Columbus L, and Criss AK. Cellular Microbiology. 17:648 – 665 (2015).
Tuning micelle dimensions and properties with binary surfactant mixtures. OliverRC, Lipfert, Fox DA, LoRH, KimJJ, DoniachS, Columbus L. Langmuir. 30:13353 – 13361 (2014).
Mapping membrane protein dynamics: a comparison of site-directed spin labeling to NMR 15N-relaxation measurements. Lo RH, Kroncke BM, Solomon T, Columbus L. Biophysical Journal.107:1697 – 1702 (2014).
Structure of the Neisserial Outer Membrane Protein Opa60: Loop Flexibility Essential to Receptor Recognition and Bacterial Engulfment. Fox DA, Larsson P, Lo RH, Kroncke BM, Kasson PM, Columbus L. J Am Chem Soc. 136:9938-9946 (2014).
James N. Demas
Education
B.S. University of New Mexico, 1964
Ph.D. University of New Mexico, 1970
National Science Foundation Postdoctoral Fellow,
University of Southern California, 1970-71
Photochemistry and Photophysics of Transition Metal Complexes
Professor Demas is not currently accepting graduate students.
Molecules excited by light lose energy by emission of light, transfer of energy or an electron to other molecules, photochemistry, and non-destructive radiationless processes. Solar energy conversion, chemical analysis, and light intensity measurements require information from the study of these processes. The processes can be highly sensitive to environmental factors such as solvent and interactions with organized media such as micelles, cyclodextrins, membranes, proteins, and DNAs. In addition, luminescence properties are very sensitive to the environment in polymer-supported sensors. We are elucidating the nature of these processes, correlating properties with molecular structure and environment, and developing new chemical, instrumental, and mathematical tools for studying these processes.
Our work includes:
- Design, synthesis and characterization of highly luminescent Os, Ir, Re, and Ru complexes.
- Evaluating photochemical properties, excited state ordering, and paths of energy loss.
- Fundamental and applied studies of interactions of photosensitizers with polymers, micelles, membranes and other organized media.
- Developing new luminescence-based sensors (e.g., oxygen, pH, metal ion).
- Design and utilization of metal complexes as probes of the structure and dynamics of organized media such as DNAs and membranes.
- Instrumental and theoretical developments in ultrasensitive, multicomponent fluorometric analyses.
An example of an analytical sensor is shown in the figure.
The photoluminescence of a tris(4,7-diphenyl-1,10-phenanthroline)ruthenium (II) complex in a polymer film is shown while the film is being breathed over. The luminescence is quite sensitive to deactivation by oxygen, and the luminescence intensity is a direct measure of the oxygen in the subject’s breath. Less oxygen yields more luminescence. The region immediately after the subject held his breath is revealing.
Recent Publications
Aromatic difluoroboron β-diketonate complexes: effects of π-conjugation and media on optical properties. Xu S, Evans RE, Liu T, Zhang G, Demas JN, Trindle CO, Fraser CL. Inorg Chem. 52:3597-610 (2013).
Viscosity and temperature effects on the rate of oxygen quenching of tris-(2,2′-bipyridine)ruthenium(II). Reynolds EW, Demas JN, DeGraff BA. J Fluoresc. 23:237-41 (2013).
Environmental sensitivity of Ru(II) complexes: the role of the accessory ligands. Dixon EN, Snow MZ, Bon JL, Whitehurst AM, DeGraff BA, Trindle C, Demas JN. Inorg Chem. 51:3355-65 (2012).
Photophysical and analyte sensing properties of cyclometalated Ir(III) complexes. Leavens BB, Trindle CO, Sabat M, Altun Z, Demas JN, DeGraff BA. J Fluoresc. 22:163-74 (2012).
Laser phosphoroscope and applications to room-temperature phosphorescence. Payne SJ, Zhang G, Demas JN, Fraser CL, Degraff BA. Appl Spectrosc. 65:1321-4 (2011).
Kateri H. DuBay
Education
B.S. Georgetown University, 2002
M.Phil. Cambridge University, 2004
Ph.D. UC Berkeley, 2009
Postdoctoral Scholar, UC Berkeley, 2009-2010
Postdoctoral Research Scientist, Columbia University, 2010-2013
The design of self-assembling nanomaterials stands as one of the great challenges in modern molecular science. The DuBay group employs theoretical and computational tools to address this challenge through investigations that lie at the intersection of soft condensed matter physics, polymer chemistry, biophysics, and nanomaterials.
At these very small length scales, the effects of thermal fluctuations, entropy, energy, and kinetics are often comparable in magnitude, rendering materials highly sensitive to perturbations such as chemical doping and environmental changes. While a wide variety of useful structures can be made via self-assembly within a static environment by precisely tuning the interactions between assembling components, environmental controls give us the means to advance beyond the limitations of such endeavors. Biological systems provide a host of examples, demonstrating the remarkable complexity and high responsivity of materials formed via environmentally-directed assembly. Specifically our group looks at assembly within environments that vary either in space, such as in the presence of a chemical gradient, or in time, such as in response to biological signaling.
Given the physical length-scales of the systems we study and the time-scale over which they evolve, we design theoretical models to capture the essential physics of the studied phenomenon. Such schematic models leave out unnecessary details in order to isolate the factors of interest and enable us to probe more directly the fundamental questions surrounding the emergence of order and responsivity within the studied nanoassemblies.
An improved understanding of the rules governing assembly in these environments will yield novel insights into the formation of functional biomaterials as well as information useful for improving light harvesting, drug-delivery, environmental-sensing, and material fabrication; countless technological innovations await the ability to rationally design artificially-ordered and environmentally-responsive nanomaterials.
Recent Publications
Construction of Donor-Acceptor Polymers via Cyclopentannulation of Poly (arylene ethynylene)s. X Zhu, S.R. Bheemireddy, S.V. Sambasivarao, P.W. Rose, R. Torres Guzman, A.G. Waltner, K.H. DuBay, and K.N. Plunkett. Macromolecules, 49 (1), 127-133 (2016).
Fluctuations within Folded Proteins: Implications for Thermodynamic and Allosteric Regulation. K.H. DuBay, G.R. Bowman, P.L. Geissler, Accounts of Chemical Research, 48 (4), pp 1098–1105 (2015).
A First-Principles Polarized Raman Method for Determining Whether a Uniform Region of a Sample is Crystalline or Isotropic. A.L. Weisman, K.H. DuBay, K.A. Willets, R.A. Friesner, The Journal of Chemical Physics 141(22), 224702 (2014).
Impact of Molecular Symmetry on Single-Molecule Conductance. E. J. Dell, B. Capozzi, K. H. DuBay, T. C. Berkelbach, J. R. Moreno, D. R. Reichman, L. Venkataraman, and L. M. Campos. J. Am. Chem. Soc. 135:32, 11724-27 (2013).
Chromophore-Controlled Self-Assembly of Highly Ordered Polymer Nanostructures. M. C. Traub, K. H. DuBay, S. E. Ingle, X. Zhu, K. N. Plunkett, D. R. Reichman, and D. A. Vanden Bout. J. Phys. Chem. Lett. 4:15, 2520-4 (2013).
Accurate Force Field Development for Modeling Conjugated Polymers. K. H. DuBay, M. L. Hall, T. F. Hughes, C. Wu, D. R. Reichman, and R. A. Friesner. J. Chem. Theory. Comput. 8, 4556-69 (2012).
Polarized Raman Spectroscopy of Oligothiophene Crystals to Determine Unit Cell Orientation. J. C. Heckel, A. L. Weisman, S. T. Schneebeli, M. L. Hall, L. J. Sherry, S. M. Stranahan, K. H. DuBay, R. A. Friesner, and K. A. Willets. J. Phys. Chem. A 116, 6804-16 (2012).
Long-Range Intra-Protein Communication Can Be Transmitted by Correlated Side-Chain Fluctuations Alone. K. H. DuBay, J. P. Bothma, and P. L. Geissler. PLoS Comput. Biol. 7:9, e1002168 (2011).

Cassandra L. Fraser
Education
B.A. Kalamazoo College, 1984
M.T.S. Harvard Divinity School, 1988
Ph.D. The University of Chicago, 1993
N.I.H. Postdoctoral Fellow, California Institute of Technology, 1993-1995
Difluoroboron dibenzoylmethane-poly(lactic acid) analogues exhibit both intense fluorescence and long-lived room temperature phosphorescence. When fabricated as nanoparticles, these simple, dual-emissive biomaterials serve as optical oxygen probes for biology and medicine, with impressive combined spatial and temporal resolution. Along with fundamental studies, the materials have been optimized with respect to fluorescence and phosphorescence emission colors, relative intensities, luminescence lifetimes, oxygen sensitivities, and fabrication. With collaborators, we developed a portable, cost-effective, laptop camera imaging system that, in conjunction with boron nanosensors, allows for dynamic, real-time, single or dual mode (ratiometric and/or lifetime) tissue oxygen imaging. We have demonstrated the utility of these materials for in vitro and in vivo optical oxygen imaging in cell, tumor, wound, vascular, brain, immunological, tissue engineering, and other contexts.
Mechanochromic Luminescence and Other Stimuli Responsive and Environment Sensitive Properties
Difluoroboron β-diketonate dyes also show surprising properties as molecular solids. For example, we showed that the difluoroboron complex of avobenzone, a simple sunscreen ingredient, has narrow bandwidth green, cyan, or blue emission depending on the solid form. Furthermore, the emission color changes when crystals are crushed or films are scratched or rubbed. Surprisingly, for thin films, the mechanochromic luminescence is reversible. For the avobenzone complex, regions where force is applied turn yellow but return to the original green-blue background color within minutes at room temperature or seconds with heating. The writing-fading process may be repeated many times. Emission colors, force responsiveness, and self-healing times may be tuned through molecular design, and self-erasing properties may be monitored with video camera imaging. These simple Scratch the Surface InksTM show promise as mechanical sensors and renewable inks for rewritable surfaces. They have even inspired creative works in music, art, and design. Other interesting properties of boron dyes, and in some cases even β-diketones absent difluoroboron, include solvatochromism, viscochromism, halochromism, aggregation induced emission, dye thickness and loading effects, and energy transfer in dye mixtures. Interestingly, some dyes are also thermally responsive and form supercooled liquids. Synthesizing new dyes, exploring their many fascinating properties, and tailoring materials for imaging and sensing in biology, medicine, and other contexts serves as the focus of our research.
Integrative Interdisciplinary Projects
Professor Fraser also has a great passion for envisioning and leading innovative interdisciplinary programs that integrate teaching, research, community engagement and creative pursuits. These projects are often inspired by materials, and environmental health and sustainability themes. They build bridges across STEM and non-STEM disciplines and engage students, faculty, and the community with thought leaders from across UVA and the globe. Examples include the UVA Page Barbour supported Transduction and Plastic/ity projects, the Carnegie Corporation funded Designing Matter Common Course, the NIH Global Health funded Metals in Medicine and the Environment, the Biomaterials Workshop, the Echols seminar Color: Across the Spectrum, and the Science, Careers and Society Forum. Professor Fraser often collaborates with artists and designers on exhibitions and performances (e.g. Chromogenic Materials, Agents of Architecture, UVA Music Technosonics Festival, UVA Art Bestiary Exchange Portfolio, Environmental Art Activism, and Time books). She also engages in design projects to establish new kinds of venues for conducting and displaying interdisciplinary work (e.g. WallSpace, Real World Chemistry Lab). New research is concerned with Anthrochemistry—chemistry of the Anthropocene, investigating the human impacts of chemistry through element, molecule, and material case studies via an interdisciplinary global systems chemistry approach. Of particular interest are ways that materials, their pathways, and processes, are mapped onto and into our bodies and intersect with our everyday lives, affecting health and wellbeing. Laws, policies, social and environmental justice, and ethics and responsibility are also considered. Creative interdisciplinary ways of communicating findings to both university and broader audiences are also of interest.
Selected Publications
Luminescent Difluoroboron β-Diketonate PLA-PEG Nanoparticles. Kerr, C.; DeRosa, C. A.; Daly, M. L.; Zhang, H.; Palmer, G. M.; Fraser, C. L. Biomacromolecules 2017, 18, 551-561.
Oxygen Sensing Difluoroboron β-Diketonate Polylactide Materials with Tunable Dynamic Ranges for Wound Imaging. DeRosa, C. A.; Seaman, S. A.; Mathew, A. S.; Gorick, C. M.; Fan, Z.; Demas, J. N.; Peirce, S. M.; Fraser, C. L. ACS Sensors 2016, 1, 1366-1373.
Mechanochromic Luminescence and Aggregation Induced Emission of Dinaphthoylmethane β-Diketones and their Boronated Counterparts. Butler, T.; Morris, W. A.; Samonina-Kosicka, J.; Fraser, C. L. ACS Appl. Mater. Interfaces 2016, 8, 1242-1251.
Polymorphism and Reversible Mechanochromic Luminescence for Solid-State Difluoroboron Avobenzone. Zhang, G.; Lu, J.; Sabat, M.; Fraser, C. L. J. Am. Chem. Soc. 2010, 132, 2160-2010.
A Dual-Emissive Materials Design Concept Enables Tumour Hypoxia Imaging. Zhang, G.; Palmer, G. M.; Dewhirst, M. W.; Fraser, C. L. Nat. Mater. 2009, 8, 747-751.
Multi-Emissive Difluoroboron Dibenzoylmethane Polylactide Exhibiting Intense Fluorescence and Oxygen-Sensitive Room-Temperature Phosphorescence. Zhang, G.; Chen, J.; Payne, S. J.; Kooi, S. E.; Demas, J. N.; Fraser, C. L. J. Am. Chem. Soc. 2007, 129, 8942-8943.
See more at Google Scholar: https://scholar.google.com/citations?user=gUlmJ6UAAAAJ&hl=en
Andreas Gahlmann
Education
B.S. University of Portland, 2005
Ph.D. California Institute of Technology, 2011
Postdoctoral Fellow, Stanford University, 2011-2014
One key area in understanding bacterial cell biology is spatiotemporal phenomena: Where, when, and how do individual biomolecules act and interact to govern the overall physiology of the cell? To answer this question, we develop new high-resolution imaging methods for 3D single-molecule localization in intact bacterial cells. In particular, we combine the resolving power of the electron microscope with the single-molecule sensitivity and specificity of fluorescence-based methods. With these tools, we can localize single biomolecules in 3D space with a precision of a few nanometers, track their motion over time, and then zoom in further to visualize how specific biomolecules combine with others to produce functioning assemblies in their native environment.
Bacteria are highly relevant to important challenges of our time. For example, the looming inability to effectively combat pathogenic bacteria with current antibiotics presents a major health concern. Finding new avenues to selectively target and alter key molecular pathways can provide us with further options for effective antibiotic drug development. Because bacteria are the smallest and arguably the simplest living organisms on the planet, they are also fundamentally interesting to study the molecular-level biology of the cell. Bacteria are able to precisely regulate protein activity throughout the intracellular space through finely tuned molecular interactions. Of particular importance are scaffolding proteins that partition the cytoplasm and provide specialized subcellular compartments for specific biochemical reactions to occur. On a smaller scale, scaffolding proteins are hypothesized to spatially organize multiple enzymes into biomolecular assemblies. Parts of these assemblies can be highly dynamic and therefore the precise architectures and the resulting functional consequences remain elusive.
Rapid progress of evolution has made the bacteria an extremely diverse and widely abundant group of single-celled organisms that affects almost every aspect of life on earth. The resulting bacterial physiological traits present a biological treasure trove that remains to be investigated with molecular resolution and, where possible, exploited to our benefit. With this in mind, we continue to push the limits of cellular imaging, as well as in situ structural characterization of biomolecular assemblies.
Recent Publications
Single-molecule tracking in live Yersinia enterocolitica reveals distinct cytosolic complexes of injectisome subunits. J. Rocha, C. Richardson, M. Zhang, C. Darch, E. Cai, A. Diepold, A. Gahlmann, Integrative Biology, 2018, 10, 502 (Cover Article)
BACT-3D: A level set segmentation approach for dense multi-layered 3D bacterial biofilms. J. Wang, R. Sarkar, A. Aziz, A. Vaccari, A. Gahlmann, S. Acton, 2017 IEEE International Conference on Image Processing (ICIP)
Bacterial Scaffold Directs Pole-Specific Centromere Segregation. J.L. Ptacin, A. Gahlmann, G.R. Bowman , A.M. Perez, A.R.S. von Diezmann, M.R. Eckart, W.E. Moerner, and L. Shapiro. Proc. Natl. Acad. Sci. USA, 2014, 111, E2046
Exploring Bacterial Cell Biology with Single-Molecule Tracking and Super-Resolution Imaging. A. Gahlmann and W.E. Moerner. Nat. Rev. Microbiol., 2013, 12, 9 (Cover Article)
Quantitative Multicolor Subdiffraction Imaging of Bacterial Protein Ultrastructures in Three Dimensions. A. Gahlmann, J.L. Ptacin, G. Grover, S. Quirin, A.R.S. von Diezmann, M.K. Lee, M.P. Backlund, L. Shapiro, R. Piestun, and W.E. Moerner. Nano Lett., 2013, 13, 987
Direct Structural Determination of Conformations of Photoswitchable Molecules by Laser Desorption-Electron Diffraction. A. Gahlmann, I-R. Lee, and A.H. Zewail. Angew. Chem. Int. Ed., 2010, 49, 6524

Robin Garrod
Education
M.Sci., University College London, UK, 2001
Ph.D., University College London, UK, 2005
Postdoctoral Fellow, The Ohio State University, 2004-2006
Astrochemistry concerns the behavior of atoms and molecules in astrophysical environments, which can include star-forming clouds and cores, and circumstellar and interstellar regions, and the solar system. The varied gas-phase chemical compositions of interstellar environments are revealed by radio-telescope observations of molecular spectral-line emission and absorption in the cm, mm and sub-mm bands. Infrared observations also indicate significant solid-phase abundances of simple hydrides, in the form of ices, which coat the sub-micron sized dust grains that permeate interstellar space. The process of star formation – which involves the heating and UV radiative processing of gas and solid-phase material alike – further encourages the production of complex organic molecules that may contribute to the store of pre-biotic material ultimately available on the surfaces of new planetary bodies.
The Garrod group develops and applies new computational techniques to the study of chemical kinetics in interstellar, star-forming, solar-system environments. A particular focus of the group is the formation and processing of simple and complex organic molecules on dust-grain surfaces and within astrophysical molecular ices. Recent new modeling efforts also include the first chemical kinetics models of solid-phase chemistry in comets.
Recent publications:
Jin, Mihwa & Garrod, Robin T.
Astrophysical Journal Supplements, 2020, 249, 26
Astrochemistry During the Formation of Stars
Jorgensen, Jes K., Belloche, Arnaud & Garrod, Robin T.
Annual Reviews in Astronomy & Astrophysics (in press), 2020
Exploring molecular complexity with ALMA (EMoCA): complex isocyanides in Sgr B2(N)
Willis, E. R., Garrod, R. T., Belloche, A. et al.
Astrophysical Journal, 2020, 636, 29
Constraining Cosmic-Ray Ionization Rates and Chemical Timescales in Massive Hot Cores
Barger, Christopher J. & Garrod, Robin T.
Astrophysical Journal, 2020, 888, 38
Simulations of Ice Chemistry in Cometary Nuclei
Garrod, Robin T.
Astrophysical Journal, 2019, 884, 69
Re-exploring Molecular Complexity with ALMA (ReMoCA): interstellar detection of urea
Belloche, A., Garrod, R. T., Müller, H. S. P. et al.
Astronomy & Astrophysics, 2019, 628, 10
Charles M. Grisham
Education
B.S. Illinois Institute of Technology, 1969
Ph.D. University of Minnesota, 1973
Biophysical Chemistry; Magnetic Resonance Spectroscopy of Complex Biological Structures
There are currently two fundamental directions to our research. In one of these, biological membranes and complex biomolecules are being studied using nuclear magnetic resonance (NMR) and electron spin resonance (ESR) techniques. Current areas of interest include two ion transporting enzymes (kidney Na,K-ATPase and muscle Ca-ATPase), and two membrane-associated signalling enzymes (protein kinase C and phospholipase C). The ATPases use the free energy of hydrolysis of ATP to transport sodium, potassium or calcium across cell membranes against large concentration gradients. Protein kinase C is an intracellular mediator of hormonal and neurotransmitter stimuli and is also the receptor for phorbol ester tumor promoters. The geometry and active site structures of these complex systems are being examined by several methods. In one, we employ paramagnetic probes, such as Mn and Gd ions, Cr-nucleotide complexes and spin label analogues of enzyme substrates and inhibitors. Such probes perturb the nuclei in their vicinity and alter the nuclear relaxation rates. Quantitation of such effects can provide distances between the probes and nuclei on the enzyme surface. Another method, transferred nuclear Overhauser enhancement, permits additional studies of the conformation of substrates and activators at the active sites of these enzymes.
One of the most interesting of these membrane-associated enzymes is the adenylyl cyclase toxin from Bordetella pertussis. Attack of host cells by this toxin results in transport of the catalytic domain of this toxin across the plasma membrane. The mechanism of this transport is not understood, but it appears to depend on a family of b-sheet helix domains in the C-terminal portion of the toxin. We are characterizing the structure and function of the b-sheet helices of this toxin by a variety of magnetic resonance techniques.
We are also examining a series of metal complexes with organic bisphosphonates as potential therapeutic agents for osteoporosis and other bone diseases. Strength and integrity of bones depend upon a balance between bone formation by osteoblasts and bone resorption by osteoclasts. Osteoclasts depend for their activity on GTP-binding proteins Rho, Rab, and cdc42, which must be prenylated to be active. Prenyl groups are synthesized in the farnesyl pyrophosphate synthase (FPS) reaction. Bisphosphonates inhibit the FPS reaction and thus inactivate osteoclasts, which then undergo apoptosis, resulting in reduced bone resorption, lower bone turnover, and a positive bone balance. Stable complexes of bisphosphonates with Cr(III), Co(III), and Rh(III) are being examined as therapeutic alternatives to the metal-free bisphosphonates.
Representative Publications
Structural Consequences of Divalent Metal Binding by the Adenylyl Cyclase Toxin of Bordetella Pertussis. Rhodes CR, Gray MC, Watson JM, Muratore TL, Kim SB, Hewlett EL, Grisham CM. Arch Biochem Biophys. 395, 169-76 (2001).
Influence of lipid on the structure and phosphorylation of protein kinase C alpha substrate peptides. Vinton BB, Wertz SL, Jacob J, Steere J, Grisham CM, Cafiso DS, Sando JJ. Biochem J. 330, 1433-42 (1998).
Intermolecular chiral recognition probed by enantiodifferential excited-state quenching kinetics. Stockman TG, Klevickis CA, Grisham CM, Richardson FS. J Mol Recognit. 9, 595-606 (1996).
31P NMR investigation of energy metabolism in perifused MMQ cells. Goger MJ, Login IS, Fernandez EJ, Grisham CM. Magn Reson Med. 3, 584-91 (1994).
Books
T. Brent Gunnoe
Education
B.A. West Virginia University, 1993
Ph.D. University of North Carolina at Chapel Hill, 1997
Postdoctoral Associate, University of Virginia, 1997 – 1999
Organometallic chemistry, inorganic chemistry, homogeneous catalysis, small molecule activation
The development of more efficient synthetic methods represents a major economic and environmental challenge for the chemical industry. With research interests that span the fields of inorganic and organic chemistry, we focus on the preparation and characterization of new transition metal complexes that are capable of activating organic molecules toward novel reactivity. By focusing on fundamental inorganic and organometallic chemistry, our efforts are directed toward the design of single-site catalysts that form the foundation of new synthetic methodologies. We apply our fundamental research toward chemical processes of relevance to the production and use of energy, the synthesis of large-scale chemicals as well as the fine chemical sector.
Petroleum distillates make up a considerable fraction of the synthetic building blocks available to the chemical industry and with a steep rise in demand, the efficient use of fossil resources is increasingly important. Carbon and hydrogen are the major elemental constituents of fossil-derived products. One area of focus for our group is the design of transition metal complexes that selectively break C–H bonds to enable reactivity towards useful products. For example, we are exploring the use of late transition metal systems for catalytic C–C bond formation reactions that proceed through transition metal-mediated C–H activation. Systems based on Ru, Pt, Rh and Ir developed in our labs catalyze the addition of aromatic (including arenes and heteroaromatic substrates) C–H bonds across the C=C bonds of olefins. The overall reactions result in arene alkylation or alkenylation. By understanding how the metal identity, metal oxidation state, and ancillary ligand impact the catalytic cycle, we can design improved catalysts.
Another area of interest is the development and study of catalysts for the selective partial oxidation of light alkanes. Natural gas is an abundant resource for chemicals, but current processes for the oxidation of light alkanes (methane, ethane, propane) to form alcohols are indirect and energy intense. As a result, distributed conversion at natural gas wellheads is not generally economical, which often results in natural gas flaring. We are studying new strategies for catalytic conversion of methane, ethane and propane to selectively generate methanol, ethanol and propanol. Our efforts are focused on both thermal and photo-driven processes.
Recently, we also have initiated projects to elucidate mechanistic details for electrocatalytic water oxidation. This is a half-reaction for overall electrocatalytic splitting of water to form dihydrogen and dioxygen, which is one strategy for the scaled conversion of abundant solar energy to chemical fuels. Electrocatalytic water oxidation is challenging due to the multi-step/multi-electron pathway as well as the common degradation of active sites to metal oxos. In collaboration with other groups, we are studying well-defined molecular catalysts, primarily based on abundant first row transition metals such as Cu and Co, including the integration of molecular active sites into conductive carbon materials.
Representative Publications
"Rhodium-Catalyzed Arene Alkenylation Using Only Dioxygen as Oxidant" Zhu, W., Gunnoe, T. B.* ACS Catal. 2020, 10, 11519-11531. DOI: 10.1021/acscatal.0c03439
"Synthesis of Stilbenes by Rhodium-Catalyzed Aerobic Alkenylation of Arenes via C–H Activation" Jia, X., Frye, L. I., Zhu, W., Gu S., Gunnoe, T. B.* J. Am. Chem. Soc. 2020, 142, 10534-10543. DOI: 10.1021/jacs.0c03935
"Use of Ligand Steric Properties to Control the Thermodynamics and Kinetics of Oxidative Addition and Reductive Elimination with Pincer-ligated Rh Complexes" Gu, S., Nielsen, R. J.*, Taylor, K. H., Fortman, G. C., Chen, J., Dickie, D. A., Goddard III, W. A.*, Gunnoe, T. B.* Organometallics 2020, 39, 1917-1933. DOI: 10.1021/acs.organomet.0c00122 (NOTE: List of "Most Read Articles" for Organometallics)
"Advances in Rhodium Catalyzed Oxidative Arene Alkenylation" Zhu, W., Gunnoe, T. B.* Acc. Chem. Res. 2020, 53, 920-936. DOI: 10.1021/acs.accounts.0c00036
"Styrene Production from Benzene and Ethylene Catalyzed by Palladium(II): Enhancement of Selectivity towards Styrene via Temperature Dependent Vinyl Ester Consumption" Jia, X., Foley, A. M., Liu, C., Vaughan, B. A., McKeown, B. A., Zhang, S., Gunnoe, T. B.* Organometallics 2019, 38, 3532-3541. DOI: 10.1021/acs.organomet.9b00349 (manuscript was selected for the issue cover)
"Mechanistic Studies of Single-Step Styrene Production Catalyzed by Rh Complexes with Diimine Ligands: An Evaluation of the Role of Ligands and Induction Period" Zhu, W., Luo, Z., Chen, J., Liu, C., Yang, L., Dickie, D. A., Liu, N., Zhang, S., Davis, R. J., Gunnoe, T. B.* ACS Catalysis 2019, 9, 7457-7475. DOI: 10.1021/acscatal.9b01480
"Catalytic Synthesis of Super Linear Alkenyl Arenes Using a Rh(I) Catalyst Supported by a "Capping Arene" Ligand: Access to Aerobic Catalysis" Chen, J., Nielsen, R. J.*, Goddard III, W. A., McKeown, B. A., Dickie, D. A., Gunnoe, T. B.* J. Am. Chem. Soc. 2018, 140, 17007-17018. DOI: 10.1021/jacs.8b07728
"Catalytic Synthesis of "Super" Linear Alkenyl Arenes Using an Easily Prepared Rh(I) Catalyst" Webster-Gardiner, M. S., Chen, J., Vaughan, B. A., McKeown, B. A., Schinski, W., Gunnoe, T. B.* J. Am. Chem. Soc. 2017, 139, 5474-5480. DOI: 10.1021/jacs.7b01165. This manuscript was highlighted in Chemical and Engineering News 2017, 95(17), 8.
"Organometallic Complexes Anchored to Conductive Carbon for Electrocatalytic Oxidation of Methane at Low Temperature" Joglekar, M., Nguyen, V., Pylypenko, S., Ngo, C., Li, Q., O’Reilly, M.E., Gray, T.S., Hubbard, W.A., Gunnoe, T. B.*, Herring, A. M.*, Trewyn, B.G.*. J. Am. Chem. Soc. 2016, 138, 116-125. This manuscript was highlighted in Chemical and Engineering 2015, 93 (43), 6; featured on cover of J. Am. Chem. Soc., selected for JACS Spotlights). DOI: 10.1021/jacs.5b06392
"A Rhodium Catalyst for Single-Step Styrene Production" Vaughan, B. A., Webster-Gardiner, M. S., Cundari, T. R.*, Gunnoe, T. B.* Science 2015, 348, 421-424. This manuscript was highlighted in Chemical and Engineering News 2015, 93 (17), 26. DOI: 10.1126/science.aaa2260
Eric Herbst
Education
A.B. University of Rochester, 1966
Ph.D. Harvard University, 1972
Postdoctoral Fellow, Harvard University and Joint Institute for Laboratory Astrophysics, University of Colorado
Professor Herbst’s major research field lies in the interdisciplinary area of molecular astronomy, which is the study of molecules throughout the universe, especially in regions in between stars known as interstellar clouds. These objects eventually collapse to form new generations of stars and planetary systems, so the molecules found in interstellar clouds are related to the molecules found in planets such as our own. Herbst is specifically interested in the chemical processes by which molecules grow, in using these chemical processes to predict the actual concentrations of molecules, and in the role of molecules in the understanding of their physical environments. His research was featured in Chemical and Engineering News, the popular journal of the American Chemical Society. A fellow of the American Physical Society and the Royal Society of Chemistry (U. K.), Herbst has won a number of international prizes including the Centenary Award of the Royal Society of Chemistry.
| Below are pictures of two astronomical objects where molecules are found. | |
| On the right is a nebula of gas and dust surrounding an old star. It is known as the “red rectangle.” |  |
| Below is an interstellar cloud so dense that no light can pass through it. | |
 |
|
Recent Publications
A New Model on the Chemistry of Ionizing Radiation in Solids: CIRIS,.Shingledecker, C. N., & Herbst, E., Phys. Chem. Chem. Phys., 19, 11043-11056 (2017)
Unified Microscopic-Macroscopic Monte Carlo Calculations of Complex Organic Molecule Chemistry in Cold Cores,. Chang Q., & Herbst, E., ApJ, 819:145(1-13) (2016)
Chemical and Physical Characterization of Collapsing Low-Mass Prestellar Dense Clouds, Hincelin, U., Commercon, B., Wakelam, BV., Hersant, F., Guilloteau, S., & Herbst, E., ApJ, 822:12(1-31) (2016)
Complex organic molecules in protoplanetary disks, Walsh, C., Millar, T. J., Nomura, H., Herbst, E., Widicus Weaver, S., Aikawa, Y., Laas, J. C., & Vasyunin, A. I., A&A, 563, A33(1-35) (2014)
Reactive Desorption and Radiative Association as Possible Drivers of Complex Molecule Formation in the Cold Interstellar Medium, Vasyunin, A. I., & Herbst, E., ApJ, 769, id. 34(1-9) (2013)
Michael Hilinski
Education
B.S. Tufts University, 2000
Ph.D. Stanford University, 2007
DOD postdoctoral fellow, University of Virginia 2009-2013
The science of organic synthesis is central to both the discovery and manufacturing of pharmaceuticals and other fine chemicals and the emergence of subdisciplines of biology that are becoming increasingly focused on phenomena at the molecular level (e.g., synthetic biology and chemical biology). Over the last half-century revolutionary advances in synthetic organic chemistry have made it possible to synthesize virtually any molecule given enough time, money, and manpower. However, this is frequently not enough since a lack of practical and cost-effective synthetic access can and does prevent promising drug leads from ever helping patients. The grand challenge for synthetic organic chemistry is therefore to advance the field of synthesis to the point where any molecule can be not only synthesized, but also synthesized in a way that minimizes the cost, time, and manpower required as well as environmental impact. Our group’s research is focused on eliminating synthetic considerations as a barrier to the discovery of new therapeutics.
Recent Publications
Organocatalytic, Dioxirane-Mediated C-H Hydroxylation under Mild Conditions Using Oxone. W. G. Shuler, S. L. Johnson, M. K. Hilinski, Org. Lett. 2017, 19, 4790–4793.
An Iminium Salt Organocatalyst for Selective Aliphatic C–H Hydroxylation. D. Wang, W. G. Shuler, C. J. Pierce, M. K. Hilinski, Org. Lett. 2016, 18, 3826–3829.
Intermolecular Electrophilic Addition of Epoxides to Alenes: [3+2] Cycloadditions Catalyzed by Lewis Acids. W. G. Shuler, L. A. Combee, I. D. Falk, M. K. Hilinski, Eur. J. Org. Chem. 2016, 3335–3338.
Chemoselective Hydroxylation of Aliphatic sp3 C–H Bonds Using a Ketone Catalyst and Aqueous H2O2. C. J. Pierce, M. K. Hilinski, Org. Lett. 2014, 16, 6504–6507.
Donald F. Hunt
Education
B.S. University of Massachusetts, 1962
Ph.D. University of Massachusetts, 1967
NIH Postdoctoral Trainee, Massachusetts Institute of Technology, 1967
Analytical Biochemistry
The goal of our research is to develop new methods and instrumentation for the structural characterization of proteins and their post-translational modifications at the low femtomole/attomole level and to apply these new methods to important structural problems in cell biology and immunology. Towards this end, we have pioneered the use of nanoflow HPLC in conjunction with microelectrospray ionization on ion trap and Fourier transform mass spectrometers. Briefly stated, the approach involves the use of proteolytic enzymes to convert the protein or group of proteins into a complex mixture of peptides, which are then fractionated by nanoflow-HPLC and eluted directly into the mass spectrometer. Mixtures containing thousands of different peptides can be analyzed in this manner. Protonated peptides of a particular mass are selected under computer control of the instrument, fragmented on collision with helium atoms and the resulting fragments are then separated and mass analyzed. Dissociation of the peptide ions occurs more or less randomly at each of the amide bonds in the molecules to produce a collection of fragments. The mass difference between two fragments differing by a single amino acid defines the mass and thus the identity of the extra residue in the longer fragment. Peptide sequence analysis is performed routinely at the femtomole and low attomole levels on the ion trap and Fourier transform instruments, respectively. Mass spectra acquired in the above manner can also be used to search databases and to identify known proteins. Currently, this approach is the most sensitive method in the world for protein characterization.
Our research focuses on two major applications of the above technology. The first involves identifying peptides that trigger the immune system to kill diseased cells. Cytotoxic T lymphocytes (CTL) or killer cells are an arm of the immune system concerned with recognition of cells that express new antigens, proteins, as a result of viral infection or cellular transformation (cancer). Cells convey their health status to the immune system by generating fragments from each of the approximately 10,000 proteins being synthesized, loading them onto a protein carrier (MHC molecule), and transporting them to the cell surface for screening by the killer cells. CTL lyse those cells that display new fragments, antigens that are associated with a particular disease state. Identification of these antigens is the first step in the preparation of vaccines that promote immunity against the above diseases. Peptides that cause the immune system to kill melanoma and lung cancer cells, to reject bone marrow transplants to leukemia patients, and to lyse tuberculosis infected cells have been identified in the laboratory recently. Efforts are in progress to characterize the peptide antigens that (a) cause rejection of tissue transplants, (b) trigger organ or tissue destruction in such autoimmune disorders as diabetes, arthritis, and multiple sclerosis, and (c) initiate an immunological response to breast, ovarian, colorectal, lung, and prostate cancers.
The second application involves research in the field of proteomics. DNA sequence information on the human genome and that of selected organisms is now becoming available at an ever-increasing rate and will provide the starting point for the development of novel therapeutic interventions against many of the world’s diseases. The next challenge is at the level of proteomics, understanding the functions of proteins encoded by a particular genome. Presently under development are mass spectrometry methods that will facilitate differential display and quantitation of most, if not all proteins expressed by healthy vs diseased cells or cells grown in the presence or absence of drugs or other agonists. Mass spectrometry is also being used to analyze all proteins secreted by a particular cell type, to identify components of functionally active protein complexes, to probe protein-protein and protein-DNA interactions, and to locate post-translational modifications and covalently attached ligands. Recently, we have developed methods that facilitate analysis of all phosphoproteins expressed in a particular cell population.
Recent Publications
Peptide Binding Motifs of Two Common Equine Class I MHC Molecules in Thoroughbred Horses, Bergman T, Lindvall M, Moore E, Sidney J, Miller D, Talmadge R, Myers P, Shabanowitz J, Osterreider N, Peters B, Hunt DF, Antczak DF, Sette A, Immunogenetics, 2017 May; 69(5):351-358. PMCID:PMC 5555743.
Canonical and Cross–reactive Binding of NK Cell Inhibitory Receptors to HLA-C Allotypes is Dictated by Peptides Bound to HLA-C. Sim MJ, Malaker SA, Khan A, Stowell JM, Shabanowitz J, Peterson ME, Rajagopalan S, Hunt DF, Altmann DM, Long EO, Boyton RJ, Front Immunol 2017 Mar 14(8);193. Doi:10.3389/fimmu.2017.00193.eCollection 2017 PMCID:PMC 5348643.
The Antigenic Identify of Human Class I MHC Phosphopeptides is Critically Dependent Upon Phosphorylation Status, Mohammed F, Stones DH, Zarling AL, Willcox CR, Shabanowitz J, Cummings KL, Hunt DF, Cobbold M, Engelhard VH, Willcox BE, Oncotarget J, 2017; April 8 (33):54160-54172. PMID: 28903331.
Front-End Electron Transfer Dissociation Coupled to a 21 Tesla FT-ICR Mass Spectrometer for Intact Protein MS/MS Analysis, Weisbrod DR, Kaiser NK, Early L, Mullen C, Syka JEP, Dunyach JJ, Anderson LC, English AM, Blakney GT, Shabanowitz J, Hendrickson CL, Marshall AG, Hunt DF, J Amer Soc Mass Spectrom, 2017;Jul 18: PMID 28721671.
Shared Peptide Binding Specificities of HLA Class I and Class II Alleles Associate with Cutaneous Nevirapine Hypersensitivity and Identify Novel Risk Alleles, Pavlos R, McKinnon EJ, Ostrov DA, Peters B, Buus S, Koelle D, Chopra A, Rive C, Redwood A, Restrepo S, Bracey A, Kaever T, Myers, P, Speers E, Malaker SA, Shabanowitz J, Jing Y, Gaudieri S, Hunt DF, Carrington M, Haas DW, Mallal S, Phillips EJ, Sci Rep, 2017; 7(1):8653.doi:10.1038/s41598-017-08876-0. PMID: 28819312.
James P. Landers
Education
B.S. University of Guelph, (Canada) 1983
Ph.D. University of Guelph, (Canada) 1988
Canadian Medical Research Council Fellow, Mayo Clinic, 1991
Polyethylene Terephthalate Microdevices
Our research group has developed a technique for fabricating microfluidic devices with complex multilayer architectures using a laser printer, a CO2 laser cutter, an office laminator, and common overhead transparencies as a printable substrate via a laser print/cut and laminate (PCL) methodology. The printer toner serves three functions; (1) it defines the microfluidic architecture, (2) acts as the bonding agent, and (3) provides printable, hydrophobic "valves" for fluidic flow control. Using common graphics software, the protocol produces microfluidic devices with a design-to-device time of ~40 min. Devices of any shape can be generated for an array of multistep assays with colorimetric detection of molecular species ranging from small molecules to proteins. The simplicity of the protocol, availability of the equipment and substrate and cost-effective nature of the process make microfluidic devices available to those who might benefit most from expedited, microscale chemistry.
Figure 1. A microfluidic chip designed to dispense sample and mix reagents by rotating at varying speeds. This specific device is used to measure albumin concentration, white blood cell count, and hematocrit in whole blood.
Biological, Bioanalytical and Clinical Chemistry
Almost every aspect of the biochemical, biomedical and clinical sciences involves separation of species in complex matrices. Electrophoresis has been a benchmark technique for separation and characterization of biologically-active species. Instead of using conventional slab gel electrophoretic approaches, electrophoresis in micron-scale capillaries using applied fields as high as 30,000 volts, results in unprecedented resolution with unique selectivities and short analysis times. As a result of the microscalar nature of the capillary, only microliters of reagent are consumed by analysis with only a few nanoliters of samples injected for analysis. These characteristics, as well as the ability for on-line detection with laser-induced fluorescence sensitivities in the attomole (10-18 moles) range, made capillary electrophoresis (CE) appealing as a replacement for electrophoretic gels in the biomedical and clinical arenas. We have demonstrated the potential impact of CE on clinical diagnostics through the development of new CE-based assays for measuring kidney function, detecting multiple sclerosis and viral infections, screening for lymphoma, as well as for diagnosing drug abuse and alcoholism.
 Figure 1. A – Schematic of capillary electrophoresis instrumentation. B – CE separation of human serum for the diagnosis of alcoholism.
Figure 1. A – Schematic of capillary electrophoresis instrumentation. B – CE separation of human serum for the diagnosis of alcoholism.
While the diagnostic impact of standard CE technology is clear, an alternative platform for electrophoresis in microscalar structures has evolved in the form of microchip electrophoresis. The use of microfabricated glass devices containing etched capillary-like channels provides an electrophoretic platform akin to CE but with more flexibility. “Microchip electrophoresis” allows for analysis times to be decreased by an order of magnitude over times achievable by CE (as fast as 10-200 seconds) and two orders of magnitude faster than gel electrophoresis. This provides obvious value to clinical diagnostic laboratories in terms of more rapid turn around time and capability for high throughput screening. We have demonstrated this with the detection T-cell and B-cell lymphoma in a separation remarkably faster than with conventional means.
 Figure 2. Demonstration of microchip electrophoresis as a technique for rapid diagnosis of T-Cell lymphoma. Sample T1 shows a negative sample, which is represented by the smear after 100 seconds of separation. T4 is a positive sample with a sharp peak.
Figure 2. Demonstration of microchip electrophoresis as a technique for rapid diagnosis of T-Cell lymphoma. Sample T1 shows a negative sample, which is represented by the smear after 100 seconds of separation. T4 is a positive sample with a sharp peak.
With a program focused on the application of miniaturized electrophoretic technology to the clinical and forensic sciences, our current efforts involve broadening the scope of applications for microchip technology. This involves addressing issues associated with integrating functions other than "separation" onto microchips. For example, we are focused on defining approaches for integrating DNA sample preparation into microchips. PCR amplification of DNA carried out using infrared-mediated thermocycling for rapid on-chip amplification and rapid DNA extraction using microchamber-bound solid phases are two examples of our integration efforts.
 Figure 3. A – Demonstration of IR-mediated PCR in a polyimide microchip. Total time necessary for thermocycling was 220 seconds. B – Elution profile of DNA in mSPE chip.
Figure 3. A – Demonstration of IR-mediated PCR in a polyimide microchip. Total time necessary for thermocycling was 220 seconds. B – Elution profile of DNA in mSPE chip.
The successful integration of DNA extraction and amplification will lead to the development of an “Integrated Diagnostic” or ID-chip, which we ultimately hope will improve laboratory medicine. Efforts are also underway to 1) define better detection systems using acoustic-optic technology, 2) develop multichannel devices for high-throughput analysis using this optical technology, 3) explore proteomic aspects of disease using multi-dimensional microchips for protein separations, and 4) apply the relevant methods to forensic applications.
Recent Publications
Simultaneous metering and dispensing of multiple reagents on passivelycontrolled microdevice solely by finger pressing. Xu K, Begley M, Landers JP. Lab on a Chip. 15: 867-876 (2015).
Integrated sample-in-answer-out microfluidic chip for Rapid HumanIdentification by STR analysis. Le Roux D, Root B, Reedy C, Hickey J, Scott O, Bienvenue J, Landers JP, Chassagne L, Mazancourt P. Lab on a Chip. 14:4415-4425 (2014).
DNA Analysis Using an Integrated Microchip for Multiplex PCRAmplification and Electrophoresis for Reference Samples. Le Roux D, Root B, Reedy C, Hickey J, Scott O, Bienvenue J, Landers JP, Chassagne L, Mazancourt P. Analytical Chemistry. 86:8192-8199 (2014).
Rapid, cost-effective DNA quantification via a visually-detectableaggregation of superparamagnetic silica-magnetite nanoparticles. Liu Q, Li J, Liu H, Tora I, Ide M, Lu J, Davis R, Green D, Landers JP. Nano Research. 7:755-764 (2014).
Dual-force aggregation of magnetic particles enhances label-freequantification of DNA at the sub-single cell level. Nelson D, Strachan B, Sloane H, Li J, Landers JP. Analytica Chimica Acta. 819:34-41 (2014).
Enhanced recovery of spermatozoa and comprehensive lysis of epithelialcells from sexual assault samples having a low cell counts or aged up to one year. Loundsbury J, Nambia S, Karlsson A, Cunniffe H, Norris J, Ferrance J, Landers JP. Forensic Science International: Genetics. 8:84-89 (2014).

John S. Lazo
Education
B.A. Johns Hopkins University, 1971
Ph.D. University of Michigan, 1976
The pharmacological mechanism of action of small molecules and on the fundamental biological role of protein tyrosine phosphatases in disease.
 Our laboratory is currently focused on two major topics: (1) discovering and characterizing novel small molecules that could lead to treatments of cancer, Alzheimer’s disease, ionizing radiation exposure and neglected diseases, and (2) validating the fundamental biological role of protein tyrosine phosphatases in cancer.
Our laboratory is currently focused on two major topics: (1) discovering and characterizing novel small molecules that could lead to treatments of cancer, Alzheimer’s disease, ionizing radiation exposure and neglected diseases, and (2) validating the fundamental biological role of protein tyrosine phosphatases in cancer.
We use a variety of platforms to seek new small molecules for human disease. These include computational modeling, high throughput target-based in vitro screening and phenotypic screening of small molecule and small interfering RNA libraries. We maintain several automated liquid handling devices and small molecule libraries for the purpose of exploring various areas of chemical space for bioactive compounds. We have been using human pluripotent cells as a model for radiation injury and mitigation.
 A second major research project focuses on investigating how the dual specificity, protein tyrosine phosphatases, such as Cdc25B and phosphatase of regenerating liver PTP4A3, control cell proliferation, migration, invasion, and survival using both molecular biological and pharmacological approaches and on applying chemical biological methodologies to the discovery of new chemical probes and potential therapeutics. We currently have developed the first well-characterized, conditional PTP4A3 knockout mouse model to investigate the role of this unique protein in colorectal tumorigenesis and tumor angiogenesis. We are seeking to identify the endogenous substrates for PTP4A3 in tumor and endothelial cells using proteomic and informatics approaches. We have discovered several potent and specific small molecule inhibitors of these protein phosphatases and are investigating their pharmacological properties.
A second major research project focuses on investigating how the dual specificity, protein tyrosine phosphatases, such as Cdc25B and phosphatase of regenerating liver PTP4A3, control cell proliferation, migration, invasion, and survival using both molecular biological and pharmacological approaches and on applying chemical biological methodologies to the discovery of new chemical probes and potential therapeutics. We currently have developed the first well-characterized, conditional PTP4A3 knockout mouse model to investigate the role of this unique protein in colorectal tumorigenesis and tumor angiogenesis. We are seeking to identify the endogenous substrates for PTP4A3 in tumor and endothelial cells using proteomic and informatics approaches. We have discovered several potent and specific small molecule inhibitors of these protein phosphatases and are investigating their pharmacological properties.
Recent Publications
Targeted deletion of the metastasis-associated phosphatase Ptp4a3 (PRL-3) suppresses murine colon cancer. Zimmerman, M.W., Homanics, G.E. and Lazo, J.S. PLoS One, 8:e58300 (2013).
Effector kinase coupling enables high-throughput screens for direct HIV-1 Nef antagonists with antiretroviral activity. Emert-Sedlak, L.A., Narute, P., Shu, S.T., Poe, J.A., Shi, H., Yanamala, N., Alvarado, J.J., Lazo, J.S., Yeh, J.I., Johnston, P.A., and Smithgall, T.E. Chem Biol. 20:82-91 (2013).
Phenotypic screening reveals topoisomerase I as a breast cancer stem cell therapeutic target. Zhang,F., Rothermund, K, Gangadharan, S.B., Pommier, Y., Prochownik, E.V., and Lazo, J.S. Oncotarget. 3:998-1010 (2012).
Alkylation sensitivity screens reveal a conserved cross-species functionome. Svilar, D., Dyavaiah, M., Brown, A.R., Tang, J., McDonald, P.R., Shun, T. Y., Wang, X-H., Lazo, J.S., Pollack, I.F., Begley, T.J. and Sobol, R. W. Mol Cancer Res., 11:1683-1692 (2012).
Discovery of diverse small molecule chemotypes with cell-based PKD1 inhibitory activity. Sharlow, E.R., Mustata Wilson, G., Close, D., Leimgruber, S., Tandon, M., Reed, R.B., Shun, T.Y., Wang, Q.J., Wipf, P., and Lazo, J.S. PLoS One 6:e25134 (2011).
Compound acquisition and prioritization algorithm for constructing structurally diverse compound libraries. Ma, C., Lazo, J.S, and Xie, X.Q. ACS Comb Sci. 13:223-231 (2011).
Awards and Honors
Kevin K. Lehmann
Education
B.S. Cook College, Rutgers University, 1977
Ph.D. Harvard University, 1983
Junior Fellow, Harvard Society of Fellows, Harvard University, 1983
Ultrasensitive Spectroscopy
There are many problems of both fundamental and of practical importance that requires measurement of extremely low concentrations of certain impurities. Molecular spectroscopy provides one approach that excels in the high specificity provided by the detailed structure in the spectrum, particularly for molecules in the gas phase. Lehmann’s group has been working on the development of new trace sensors, largely based upon the method of cavity ring-down spectroscopy (CRDS). In CRDS, one forms a stable optical cavity using mirrors with reflectivity > 99.99 percent and observes absorption of a sample contained inside the cavity by an increase in the rate of decay of light that is trapped between the mirrors. Sample absorption as low as 1 part in 109 per pass of the cell can be measured in this way. The Lehmann group pioneered the use of low cost and rugged diode lasers developed for the telecom industry in CRDS and has demonstrated detection of a number of small molecules, such as H2O, NH3, NO, and CH4 at levels below one part per billion in a sample gas. Tiger Optics, Inc. is now selling instruments based upon this work to several industries.
A current focus of the research is development of a version of CRDS that exploits near-resonant two-photon IR absorption of a low-pressure gas. A low-loss optical cavity can enhance the intensity of light up to ten thousand times over the intensity of the laser used to excite the cavity. Two-photon absorption of counter propagating photons is with out Doppler Broadening, resulting in transitions with widths ~103 times narrower than the Doppler width of transitions. While the spectrum of polyatomic molecules often have crowded spectra due to the large number of thermally populated rotational and vibrational states, the two-photon spectrum is dominated by only a few transitions where there is a pair of transitions, sharing an intermediate level, that are very nearly at the same frequency. For modest size polyatomic molecules, the two-photon absorption spectrum has less than 0.1% as many strong lines at the one photon spectrum. The combination of the small number and small widths of the lines mean that the typical two-photon spectrum is ~106 times more selective than the one photon spectrum in the same spectral region. In addition, the two-photon loss of a cavity can be separated from linear loss contributions to the cavity from mirrors, scattering, and one-photon absorption, which results in increased sensitivity as well.
Double Resonance Spectroscopy
There are many situations, such as in combustion and for “Hot Jupiter” Exoplanets, where simulation of spectra of hot samples of polyatomic molecules is needed. Traditionally, such spectra are difficult to study experimentally due to spectral congestion and sample decomposition. Great strides have been made in theoretical modeling of such spectra but these theoretical spectra need to be tested experimentally to assess their accuracy, particularly with respect to transitions starting from excited vibrational states. The Lehmann group, in collaboration with a group in the Univ. of Umea in Sweden, has developed a novel double resonance method that involves using a narrow bandwidth infrared Optical Parametric Oscillator to pump a single ro-vibrational transitions of a molecule (so far we have focused on methane) and then probe absorption transitions from this excited state with a frequency comb, which allows us to simultaneously detect absorption has tens of thousands of discrete frequencies, each determined with ~1 KHz frequency accuracy. As the pump laser only pumps molecules with a narrow width of Doppler Shift, the probe transitions are sub-Doppler with a width of only a few MHz.
Meta-Science
There is currently a “replication crisis” is several fields of science, such as psychology and cancer research, where it has been found that a majority of published experiments fail to give the reported results when carefully repeated by independent laboratories. There are more isolated reports that a substantial fraction of chemical syntheses cannot be reproduced using only what is in the published papers. Spectroscopy is most often more about measurement than hypothesis testing and has substantially redundancy in that many transitions are fit to high accuracy with a Hamiltonian with a limited number of constants. However, the accuracy of derived molecular parameters are often dependent upon the details of statistical analysis and poorly understood model errors. The lab is currently doing systematic investigations the historical reproducibility of spectroscopic constants and the molecular properties derived from them, such as equilibrium structures.
Recent Publications
- Simon Lobsiger, Cristobal Perez, Luca Evangelisti, Kevin K. Lehmann, and Brooks H. Pate, Molecular Structure and Chirality Detection by Fourier Transform Microwave Spectroscopy, J. Phys. Chem. Lett. 6, 196-200 (2015)
- Y. Chen, P. Mahaffy, V. Holes, J. Burris, P. Morey, K.K. Lehmann, B. Sherwood Lollar, G. Lacrampe-Couloume, and T.C. Onstott, Near Infrared Cavity Ring-Down Spectroscopy for Isotopic Analysis of CH4 on Future Martian Surface Missions, Planetary and Space Sciences 105, 117-122 (2015).
- Vitali I. Stsiapura, Vincent K. Shuali, Benjamin M. Gaston, and Kevin K. Lehmann, Detection of S‑Nitroso Compounds by Use of Midinfrared Cavity Ring-Down Spectroscopy, Analytical Chemistry 87, 3345-53 (2015).
- Z Meng, G.I. Petrov, S Cheng, J.A. Jo, K.K. Lehmann, V.V. Yakovlev, M.O. Scully, Lightweight Raman Spectroscope using time-correlated photon counting detection, Proceedings of the National Academy of Sciences, 112, 12315-12320 (2015).
- Chen, Y., K. K. Lehmann, Y. Peng, L. M. Pratt, J. R. White, S. B. Cadieux, B. S. Lollar, G. Lacrampe-Couloume and T. C. Onstott, Hydrogen Isotopic Composition of Arctic and Atmospheric CH4 Determined by a Portable Near-Infrared Cavity Ring-Down Spectrometer with a Cryogenic Pre-Concentrator, Astrobiology 16(10): 787-797. (2016).
- Lehmann, K. K., Influence of resonant collisions on the self-broadening of acetylene, Journal of Chemical Physics 146(9): 094309 (2017).
- Seifert, N. A., D. P. Zaleski, R. Fehnel, M. Goswami, B. H. Pate, K. K. Lehmann, H. O. Leung, M. D. Marshall and J. F. Stanton, The gas-phase structure of the asymmetric, trans-dinitrogen tetroxide (N2O4), formed by dimerization of nitrogen dioxide (NO2), from rotational spectroscopy and ab initio quantum chemistry, Journal of Chemical Physics 146(13): 134305 (2017).
- K.K. Lehmann, Theory of Enantiomer-Specific Microwave Spectroscopy, in Frontiers and Advances in Molecular Spectroscopy, pgs 713-743 (2018). Edited by Jaan Laane, Elsevier publisher.
- K.K. Lehmann, Influence of spatial degeneracy on rotational spectroscopy: Three-wave mixing and enantiomeric state separation of chiral molecules, Journal of Chemical Physics 149(9): 094201 (2018).
- J. Karhu, K.K. Lehmann, M. Vainio, M. Metsala, and L. Halonen, Step-modulated decay cavity ring-down, Optics Express 26(22): 094201 (2018)
- K. K. Lehmann, Resonant enhanced two-photon cavity ring-down spectroscopy of vibrational overtone bands: a proposal, Journal of Chemical Physics 151: 144201(2019), https://doi.org/10.1063/1.5122988.
- Gang Zhao, D. Michelle Bailey, Adam J. Fleisher, Joseph T. Hodges, and Kevin K. Lehmann, Doppler-Free two-photon cavity ring-down spectroscopy of a nitrous oxide (N2O) vibrational overtone transition, Physical Review A 101, 062509 (2020).
- Kevin K. Lehmann, Optical cavity with intracavity two-photon absorption, Journal of the Optical Society of America B 37, 3055 (2020).
- Aleksandra Foltynowicz, Lucile Rutkowski, Isak Silander1, Alexandra C. Johansson, Vinicius Silva de Oliveira, Ove Axner, Grzegorz Soboń, Tadeusz Martynkien, Paweł Mergo, and Kevin K. Lehman, Sub-Doppler Double-Resonance Spectroscopy of Methane Using a Frequency Comb Probe, to be published in Physical Review Letters.
- Aleksandra Foltynowicz, Lucile Rutkowski, Isak Silander1, Alexandra C. Johansson, Vinicius Silva de Oliveira, Ove Axner, Grzegorz Soboń, Tadeusz Martynkien, Paweł Mergo, and Kevin K. Lehman, Measurement and assignment of double-resonance transitions to the 8900--9100 cm-1 levels of methane, to be published in Physical Review A.

Timothy L. Macdonald
Education
B. Sc. University of California, Los Angeles, 1971
Ph. D. Columbia University, 1975
NIH Postdoctoral Fellow, Stanford University, 1975-77
Bioorganic and Synthetic Organic Chemistry
The unifying theme of my research program is the application of organic chemical theory and technique to the investigation of problems in biology. Our longstanding interest has been in elucidating the molecular interactions and mechanisms of small molecule-protein interactions. Such interactions are fundamental to physiology, pharmacology, and toxicology and understanding these processes in molecular terms is essential for predicting modes of metabolism and developing new drugs. Our efforts are currently directed at several rather broad areas: elucidation of the molecular pharmacology of ligand-receptor interactions; identification of the molecular processes underlying idiosyncratic drug reactions; determination of the chemical mechanisms of the oxidative transformation of foreign and endogenous compounds by monooxygenase and dioxygenase enzymes; and characterization of the molecular mechanisms underlying the neurotoxicity of small molecules, metal ions, and reactive oxygen species.
Current studies of ligand-receptor interactions include the lysophospholipid signaling system, the colchicinoid site on tubulin, and the ternary drug-DNA-topoisomerase II complex responsible for the cytotoxicity of this class of anticancer agents. Lipid phosphoric acid mediators, such as lysophosphatidic acid and sphingosine-1-phosphate, have a range of biological properties mediated through at least eight G-protein coupled receptors of the EDG family. The cellular biology of these receptors and the physiological mechanisms controlling the processing of the lysophospholipids is beginning to emerge. We are undertaking a set of investigations directed at elucidating the molecular pharmacology of this receptor family and at utilizing this knowledge to target the modulation of lysophospholipid signaling to treat human disease. Specifically, our studies of the lysophospholipid autocoids have been directed at identifying the molecular determinants of the lipid mediator and the receptor that have been proposed to be critical to activity and at developing receptor selective agents for the EDG receptors.
We also have a long-standing interest in understanding ligand-receptor interactions which form ternary DNA-drug complexes with a DNA processing enzyme, and a small molecular ligand. Such complexes are a common theme for a diverse variety of “effectors” of cellular function, including hormone regulators of cellular transcription and translation, and of many antineoplastic agents. We have been examining the interaction of the representative antitumor agent, etoposide, which forms a ternary DNA-drug complexes with DNA topoisomerase I. Another area of research involves elucidation of the molecular mechanism through which colchicine interacts with its target, tubulin. These programs have fully integrated synthetic studies of rationally designed molecules and mechanistic studies of the processes through which these agents interact with their targets. Our studies will advance the knowledge of antitumor drug development for these classes of agents and culminate in the design and synthesis of fundamentally new structural classes of inhibitors of these critical anticancer targets.
An additional area of research is the molecular and cellular mechanisms by which idiosyncratic drug reactions occur. These reactions are the source of a range of toxic reactions to therapeutic agents and are thought to be mediated through the confluence of risk factors associated with drug bioactivation to reactive species, the formation of specific protein antigens and subsequent immune response. A knowledge of the chemical and immunological basis for idiosyncratic reactions would enable a protocol for prospectively identifying individual patient risk or susceptibility to particular agents or drug classes. We have previously investigated the mechanism of blepharoconjunctivitis and dermatitis associated with the use of the antiglaucoma drug, apraclonidine. Our current focus is on the mechanism underlying the aplastic anemia associated with the use of the anti-epileptic agent, felbamate.
Our research is additionally exploring the mechanistic and etiologic relationships between neurotoxicity and aberrant CNS processing of foreign and endogenous agents. The brain is the site of multiple, chronically progressive and clinically devastating diseases in which specific neuronal populations undergo neurodegeneration. An environmental contribution has been implicated in the etiology of many of these diseases, including Parkinson’s disease, motoneuron disease, and Alzheimer’s disease. In one avenue of study, we are examining the potential for the formation of endogenous or exogenous toxins by oxidative enzymes that have been localized to particular neuronal populations, such as the cytochromes P450 and their associated reductases. Through these studies, we hope to ascertain whether this enzyme class represents a primary basis for the etiology or treatment of these neurodegenerative diseases.
Recent Publications
A model for the regulation of T-type Ca(2+) channels in proliferation: roles in stem cells and cancer. Gray LS, Schiff D, Macdonald TL. Expert Rev Anticancer Ther. 13:589-95 (2013).
Cost-effective and Large-scale synthesis of 16:0 Lysophosphatidic Acid. East JE, Macdonald TL. Synth Commun. 42:3614-3618 (2012).
Amixicile, a novel inhibitor of pyruvate: ferredoxin oxidoreductase, shows efficacy against Clostridium difficile in a mouse infection model. Warren CA, van Opstal E, Ballard TE, Kennedy A, Wang X, Riggins M, Olekhnovich I, Warthan M, Kolling GL, Guerrant RL, Macdonald TL, Hoffman PS. Antimicrob Agents Chemother. 56:4103-11 (2012).
Development of a phosphatase-resistant, L-tyrosine derived LPA1/LPA3 dual antagonist. East JE, Carter KM, Kennedy PC, Schulte NA, Toews ML, Lynch KR, Macdonald TL. Medchemcomm. 2:325-330 (2011).
Sphingosine kinase type 1 inhibition reveals rapid turnover of circulating sphingosine 1-phosphate. Kharel Y, Mathews TP, Gellett AM, Tomsig JL, Kennedy PC, Moyer ML, Macdonald TL, Lynch KR. Biochem J. 440:345-53 (2011).
Charles Machan
Education
B.A. Washington University, 2008
Ph.D. Northwestern University, 2012
Postdoctoral Researcher, University of California, San Diego 2013-2016
The Machan Group is interested in energy-relevant catalysis, particularly at the interface of molecular electrochemistry and materials. The development of efficient and selective transformations to produce commodity chemical precursors and fuels using CO2, O2, H2, and H2O as reagents remains an ongoing challenge for the storage of electrical energy within chemical bonds. Our approach is inspired by the numerous metalloproteins capable of catalyzing kinetically challenging reactions with significant energy barriers in an efficient manner under ambient conditions. This type of reactivity is achieved through the convergent evolution of active sites with tailored coordination environments and macromolecular structures which can, among other things, transport substrates and products to and from the active site. Our research focuses on developing new inorganic complexes and materials which incorporate co-catalytic moieties, non-covalent secondary sphere interactions, and substrate relays as catalysts.
In order to characterize and optimize these systems, research in the Machan Group uses synthetic inorganic chemistry, electrochemistry, and advanced characterization techniques (spectroelectrochemistry, stopped-flow IR and UV-vis spectroscopies). This enables us to develop an understanding of electronic structure and mechanism in transformations of interest. A brief summary of current projects is listed below.
Electrochemical Reduction of Dioxygen
The reduction of dioxygen (O2) is of vital importance to energy related reactions. In biology, respiration uses O2 reduction as a thermodynamic sink, whereas fuel cells pair the oxygen reduction reaction (ORR) to H2O as a proton-dependent half-reaction to the oxidation of chemical fuels. Catalytic ORR processes mediated by molecular species continue to garner interest as models for more complex heterogeneous systems. We are interested in the understanding selective activation and reduction of O2 to H2O2 or H2O by molecular species through electrochemical, spectrochemical, and spectroelectrochemical studies. Using these studies, we are developing new ligand frameworks for aqeuous and non-aqeuous systems, some of which are models for metalloenzyme active sites.
· Electrocatalytic Reduction of Dioxygen to Hydrogen Peroxide by a Molecular Manganese Complex with a Bipyridine-Containing Schiff Base Ligand (J. Am. Chem. Soc. 2018 DOI:10.1021/jacs.7b09027)
· Dioxygen Reduction to Hydrogen Peroxide by a Molecular Mn Complex: Mechanistic Divergence between Homogeneous and Heterogeneous Reductants (J. Am. Chem. Soc. 2019 DOI:10.1021/jacs.8b13373)
· Electrocatalytic Reduction of Dioxygen by Mn(III) meso-Tetra(N-methylpyridinium-4-yl)porphyrin in Universal Buffer (Dalton Trans. 2019 DOI: 10.1039/C9DT01436E)
Molecular Electrocatalysts for the Electrochemical Conversion of Carbon Dioxide
The efficient and cost-effective catalytic reduction of CO2 using renewable energy remains a significant challenge for molecular species. Heterogeneous systems can produce highly reduced products like methane and ethylene from CO2, but these generally suffer from a lack of selectivity. The intrinsic advantage of molecular systems is the relative ease with which they may be characterized and quantified, relative to the distribution of active site morphologies that may be present in a bulk material. Using bioinspired design principles, we are investigating catalytic conversion strategies for producing CO and formic acid from CO2.
· Electrocatalytic Reduction of CO2 to Formate by an Iron Schiff Base Complex (Inorg. Chem. 2018, DOI: 10.1021/acs.inorgchem.7b02955)
· Highly Efficient Electrocatalytic Reduction of CO2 to CO by a Molecular Chromium Complex (ACS Catalysis 2020, DOI: 10.1021/acscatal.9b04687)
· Electrochemical CO2 Reduction in a Continuous Non-Aqueous Flow Configuration with [Ni(cyclam)]2+ Catalyst (Inorg. Chem. 2020 DOI: 10.1021/acs.inorgchem.9b03171)
· Electrocatalytic CO2 Reduction to Formate with Molecular Fe(III) Complexes Containing Pendent Proton Relays (Inorg. Chem. 2020 DOI: 10.1021/acs.inorgchem.9b03341)
Porous Electrocatalyst Materials
Metal-organic frameworks (MOFs) and covalently linked organic frameworks (COFs) continue to attract significant interest in materials chemistry. MOFs and COFs offer many advantages in terms of porosity and stability over more amorphous materials or zeolites. Indeed, the translation of molecular properties to bulk materials in this manner has implications for the development of electrochemically responsive films and membranes. We are focused on developing new methods for synthesizing and processing conducting and semi-conducting 2D MOF and COF materials sensitive to the chemical environment. This is primarily focused on applications in molecular detection, separation, and catalysis. A fundamental understanding of how molecular properties are translated in these systems will enable future studies focusing on other applications in energy storage and optoelectronic devices.
· Metal-Organic Frameworks as Porous Templates for Enhanced Cobalt Oxide Electrocatalyst Performance (ACS Applied Energy Materials 2019 DOI: 10.1021/acsaem.9b00127)
Publications can be accessed at "see more"
Glenn J. McGarvey
Education
B.S. University of California, Santa Barbara, 1973
Ph.D. University of California, Davis, 1977
N.I.H. Postdoctoral Fellow, California Institute of Technology, 1977-79
Molecular Glycosciences
-
Complex Glycoconjugate Synthesis
-
Carbohydrate-Based Molecular Recognition
Cell surface carbohydrates, typically conjugated to proteins and lipids, are key elements in the cellular communication that is essential to cellular organization and function in all organisms. Not only do these cell surface constituents mediate normal behavior in cells, but they may also participate in molecular recognition events that are associated with many serious human diseases, including rheumatoid arthritis, viral and bacterial infections, and cancer metastasis. This provides an extremely attractive opportunity for selective chemical intervention of cell function through the targeting of specific cell surface carbohydrate structures.
A central theme of our research is the application of synthetic organic chemistry to the preparation of appropriate carbohydrate structures in order to carry out detailed structural studies addressing their recognition properties. The highly complex structures of carbohydrates, particularly in the form of their corresponding glycoconjugates, often challenge the limits of existing synthetic technology and dictate the development of new methodology. As such, studies addressing biological carbohydrates offer opportunities for discoveries in the both the chemical and biological domains.
One for the focuses of our efforts is the development of synthetic cancer vaccines directed toward cell surface carbohydrate antigen structures. In this context, the synthesis of complex oligosaccharides is currently under investigation, as well as the development of new strategies for the assembly of unnatural glycopeptide structures that may prove useful in the assembly of effective vaccines.
In other studies, we are endeavoring to exploit the dense stereochemical and functional content of carbohydrates to generate new classes of molecules for highly selective biorecognition. In particular, C-glycoside oligosaccharide/peptide hybrids are being examined for specific cell surface and DNA recognition and binding toward the goal of making available new methods for the treatment of diseases and for the rational modification of biological function.
 Representative Publications
Representative Publications
Studies on the stereoselective synthesis of C-allyl glycosides. McGarvey GJ, LeClair CA, Schmidtmann BA. Org Lett. 10, 4727-30 (2008).
The preparation of C-glycosyl amino acids – an examination of olefin cross-metathesis. Schmidtmann FW, Benedum TE, McGarvey GJ. Tetrahedron letters. 46, 4677-4681 (2005).
Synthesis of alpha- and beta-C-glycosides of N-acetylglucosamine. McGarvey GJ, Schmidtmann FW, Benedum TE, Keizer DE. Tetrahedron letters. 44, 3775-3779 (2003).
Development of co- and post-translational synthetic strategies to C-neoglycopeptides. McGarvey GJ, Benedum TE, Schmidtmann FW. Org Lett. 4, 3591-4 (2002).

Lisa Morkowchuk
Education
B.S. Moravian College, 2008
Ph.D. Rensselaer Polytechnic Institute, 2013
Lisa Morkowchuk is an instructor for Introductory College Chemistry lecture (1410/1610) and laboratories (1411/1611). She received a B.S. in Chemistry from Moravian College in Bethlehem, Pennsylvania and a Ph.D. in Chemistry from Rensselaer Polytechnic Institute in Troy, New York. Much of her undergraduate education was presented in a guided-inquiry format, and she quickly realized the value of peer interaction in education and the depth of understanding that comes from inquiry-based learning. Implementation of these approaches can be a challenge in courses with large enrollments, but it is one way that physical institutions can provide value above that offered by online courses. Lisa strives to continually increase the proportion of “lecture” time spent utilizing non-lecture, evidence-based pedagogical techniques.
Before coming to the University of Virginia, she taught chemistry and mathematics courses at Albany College of Pharmacy and Health Sciences and at Rensselaer Polytechnic Institute. She currently lives in Charlottesville, Virginia with her partner and kids and enjoys endurance running in her free time.

Michael Palmer
Education
A.S. Casper Community College
B.S. University of Wyoming
Ph.D. University of Wyoming
Michael Palmer, Associate Director and Associate Professor, joined the Teaching Resource Center in the Fall of 2003. As an Associate Director, he presents interactive workshops locally, nationally and internationally; he regularly consults with faculty, graduate student instructors, departments, and administrative units about teaching and learning matters; and he designs and administers professional development programs, such as the TRC’s graduate student professional development program, Tomorrow’s Professor Today, and the Center’s annual Course Design Institute. His educational development research centers on teaching consultation techniques, graduate student professional development, and the impact of intense professional development activities on teacher beliefs and practices. Published accounts of his work can be found in To Improve the Academy, Practically Speaking: A Sourcebook for Instructional Consultants in Higher Education (2nd Ed, 2012; editor Kate Brinko), and Studies in Graduate and Professional Student Development. He was the 2011 Professional and Organizational Development Network in Higher Education’s (POD Network) conference co-chair and has served on the core faculty of the 2009, 2011, and 2013 New Faculty Developers Institutes. He is currently a member of the POD Network’s Core Committee, the organization’s Board of Directors, and chair of the Membership Committee.
Michael’s pedagogical interests include course design, active learning, student motivation, creative thinking, and teaching large enrollment courses, particularly in STEM disciplines. He teaches a highly interdisciplinary course on infinity and a large-enrollment, inquiry-based laboratory course for first-year chemistry students. In 2012, he won one of UVa’s All-University Teaching Awards.
Born and raised in Wyoming, Michael obtained his B.S. and Ph.D. in chemistry at the University of Wyoming in Laramie. There he won both the University of Wyoming Outstanding Dissertation Award and the Sara Jane Rhoads Award for Outstanding Research for the Ph.D. Degree in Chemistry. Upon completing his graduate studies, Michael accepted a postdoctoral research position in the Chemical Engineering Department at the University of Virginia. Michael’s research focused on environmentally and industrially important catalytic processes, from the desulfurization of petroleum feedstocks and the conversion of natural gas to liquid fuels to the selective oxidation of aromatic compounds. Published accounts of his chemical research can be found in the Journal of the American Chemical Society, Journal of Physical Chemistry B, and Organometallics.

Brooks H. Pate
Education
B.S. University of Virginia, 1987
Ph.D. Princeton University, 1992
NRC Postdoctoral Fellow (NIST, Gaithersburg), 1992-93
Broadband Rotational Spectroscopy for Chemical Analysis
The Pate lab develops instruments for molecular rotational spectroscopy and uses these instruments to solve challenging problems in chemical analysis. Molecular rotational spectroscopy uses low frequency light, typically in the microwave region of the electromagnetic spectrum, to excite transitions between the quantized energy levels that come from the rotational kinetic energy. The energy level patterns for the rotational kinetic energy are determined by the principal moments-of-inertia of the molecule and are, therefore, directly related to the mass distribution relative to the molecular center-of-mass. The fact that the measurement is connected to the mass distribution, and not just the total mass, makes molecular rotational spectroscopy ideally suited for isomer analysis.
Broadband Rotational Spectroscopy
The Pate lab has pioneered the technique of chirped-pulse Fourier transform rotational spectroscopy. The key advantage of this technique over previous instrument designs is the ability to measure a large spectral range in a single spectrum acquisition event. The method has similarities to Fourier transform nuclear magnetic resonance (NMR) spectroscopy: A high-power light pulse (the chirped pulse) creates a macroscopic polarization in the sample by aligning the molecular dipole moments. After the excitation pulse dissipates, the molecular rotational resonances are detected by the light waves coherently emitted by the rotating molecules. This coherent emission eventually decays through Doppler dephasing or collisions. The broadband free-induction decay (FID) from the rotational motion is collected using a high-speed digitizer. Sensitivity is enhanced by acquiring several measurements and co-adding the FIDs. The molecular rotational spectrum is produced by subsequent Fourier transform analysis of the FID. This measurement approach has been extended to low-frequency instruments (2-8 GHz) that are used for analysis of large molecules and to high-frequency instruments (mm-wave range) for smaller molecules such as those important in astrochemistry
Initial Applications of Broadband Rotational Spectroscopy
The first application of chirped-pulse Fourier transform rotational spectroscopy was in the field of intramolecular dynamics. In this application, the instrument is used to acquire the rotational spectrum of a highly vibrationally excited molecule that is prepared by laser excitation. The complex nuclear motion from intramolecular vibrational energy redistribution and conformational isomerization produce dynamic effects in the spectrum. For example, the coalescence of rotational spectra associated with different conformational geometries can be used to measure the energy-resolved unimolecular isomerization rate under collision free conditions.
A second area of application is the structure of molecular clusters. Clusters form in the pulsed jet expansion used to create the cold gas sample used for analysis. The aggregation is driven by non-covalent interactions such as hydrogen bonding and London dispersion forces. A challenge for the analysis of the clusters is that a wide range of cluster sizes are produced and there can be many isomeric structures for each cluster size. Rotational spectroscopy provides the highest spectral resolution of molecular spectroscopy techniques used for chemical analysis. As a result, the technique has no trouble resolving the spectra from all cluster geometries present in the sample. The application of broadband rotational spectroscopy to water clusters has dramatically increased the number of known structures, demonstrated the presence of multiple isomers for a single cluster size, and revealed the dynamics of quantum mechanical tunneling within the water cluster.
Chiral Analysis with Applications to Pharmaceutical Chemistry
The most recent work in the Pate lab is the application of broadband rotational spectroscopy to chiral analysis. The group is developing techniques for quantitative chiral analysis that uses the formation of clusters of an analyte and a small, enantiopure chiral molecule (called the chiral tag) to determine the enantiomeric excess of the sample. Using quantum chemistry modeling of the chiral tag complexes it is also possible to establish the absolute configuration of the dominant enantiomer. A key strength of rotational spectroscopy is that the measurement can simultaneously perform other analyses that are related to the synthesis of chiral molecules. For example, when the analyte has multiple chiral centers there are two types of stereoisomers: the enantiomers (left- and right-handed versions of a molecule) and diastereomers (isomers with different structures and mass distributions that are easily distinguished by molecular rotational spectroscopy). The technique can also be used to quantify regioisomers that are often present due to addition reactions that occur at other activated sites of the reagent in the reaction step. The high-resolution of rotational spectroscopy makes it possible to perform these analyses without extensive sample purification. Furthermore, cavity-enhanced rotational spectroscopy instruments can be used to monitor the synthesis of chiral molecules in real-time by sampling directly out of the reaction flask.
Representative Publications
Gordon G. Brown, Brian C. Dian, Kevin O. Douglass, Scott M. Geyer, and Brooks H. Pate, “A Broadband Fourier Transform Microwave Spectrometer Based on Chirped Pulse Excitation” Rev. Sci. Instrum. 79, 053103 (2008).
Brian C. Dian, Gordon G. Brown, Kevin O. Douglass, and Brooks H. Pate, “Measuring Picosecond Isomerization Dynamics via Ultra-broadband Fourier Transform Microwave Spectroscopy”, Science 320, 924-928 (2008).
Cristóbal Pérez, Matt T. Muckle, Daniel P. Zaleski, Nathan A. Seifert, Berhane Temelso, George C. Shields, Zbigniew Kisiel, and Brooks H. Pate, “Structures of Cage, Prism, and Book Isomers of Water Hexamer from Broadband Rotational Spectroscopy”, Science 336, 897-901 (2012).
Jeremy O. Richardson, Cristóbal Pérez, Simon Lobsiger, Adam A. Reid, Berhane Temelso, George C. Shields, Zbigniew Kisiel, David J. Wales, Brooks H. Pate, and Stuart C. Althorpe, “Concerted hydrogen-bond breaking by quantum tunneling in the water hexamer prism”, Science 351, 1310-1313 (2016).
B.H. Pate, L. Evangelisti, W. Caminati, Y. Xu, J. Thomas, D. Patterson, C. Perez, and M. Schnell, “Quantitative Chiral Analysis by Molecular Rotational Spectroscopy”, in Chiral Analysis 2nd Edition Advances in Spectroscopy, Chromatography, and Emerging Methods, P.L. Polavarapu, Editor, Elsevier (2018).
Justin L. Neill, Yuan Yang, Matt T. Muckle, Roger L. Reynolds, Luca Evangelisti, Reilly E. Sonstrom, Brooks H. Pate, and B. Frank Gupton, “Online Stereochemical Process Monitoring by Molecular Rotational Resonance Spectroscopy”, Org. Process Res. Dev. 23, 1046-1051 (2019).

Rebecca R. Pompano
Education
B.S. University of Richmond, 2005
Ph.D. University of Chicago, 2011
Postdoctoral Scholar, University of Chicago, 2011-2014
Our lab develops methods based on microfluidic culture systems, bioanalytical techniques, and spatially resolved simulations to quantify the spatiotemporal dynamics of the inflammatory cascade and develop targeted therapies. This work is part of a broad interest in the dynamics of complex biological systems. Specifically, we study the kinetics of immunity and inflammation, and we develop chemically targeted methods to control these processes in the context of vaccination, autoimmunity, and chronic inflammatory disease.
The immune system is a fascinating topic for physical scientists to study, and one where novel analytical tools can make a significant impact. The system consists of a highly structured network whose components include a set of specialized cell types and secreted signals, which organize themselves into dynamic spatial arrangements. These components interact with all of the characteristics of mathematical complexity, including nonlinearity, thresholds for activation, and multiple length scales, and they exhibit emergent behaviors that are difficult to predict from knowledge of the individual components. As a result of this complexity, protective immune responses against invading pathogens and injected vaccines are only a small perturbation away from the non-productive inflammation that characterizes autoimmunity, heart disease, Alzheimer’s disease, and solid tumors. Despite a wealth of information about individual proteins and cells in the immune system, it is challenging to predict the effects of a given stimulation of the immune system. One reason is that chemical stimulation of individual clusters of cells is still difficult to achieve in vitro or in vivo. Spatially resolved readout of secreted molecules is also difficult, unless the molecules can be fluorescently labeled. Without such tools, it remains unclear how to stop an autoimmune disease without suppressing the entire immune system, or how to design potent vaccines that work for any disease target without unwanted inflammatory side effects.
We are developing new methods to study the kinetics and the spatial behavior of cells and secreted signals during immune responses. For example, we are designing microfluidic devices to test the effects of spatial distribution and local delivery of signals. We also are developing new ways to measure the secretions of cells in living tissues with high spatial and temporal resolution. To do so, we combine activities from a variety of disciplines, including microfabrication and device design; quantitative analysis of chemical and biochemical signals using immunoassays, HPLC, mass spectrometry, and fluorescence microscopy (widefield and confocal); live cell and tissue imaging using samples from mouse models of health and disease; and, finally, spatially-resolved time-dependent numerical simulations. Eventually, we will use the information from these experiments to design spatially targeted nanoparticles that abrogate inflammation. Our goal is that the methods we develop will enable experiments that contribute to the fundamental understanding of both immunity and complex chemical kinetics, and that they will help guide the design of highly targeted vaccines and immunotherapies.
Representative Publications
Materials-based modulation of inflammation and immune responses:
The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Chen J*, Pompano RR*, Santiago FW, Maillat L, Sciammas R, Sun T, Han H, Topham DJ, Chong AS, Collier JH. Biomaterials. 2013; 34(34):8776-85. *Equal contributions.
Device design for microfluidic analyses:
Control of initiation, rate, and routing of spontaneous capillary-driven flow of liquid droplets through microfluidic channels on SlipChip. Pompano RR, Platt CE, Karymov MA, Ismagilov RF. Langmuir. 2012; 28(3):1931-41.
Toward mechanistic understanding of nuclear reprocessing chemistries by quantifying lanthanide solvent extraction kinetics via microfluidics with constant interfacial area and rapid mixing. Nichols KP, Pompano RR, Li L, Gelis AV, Ismagilov RF. J Am Chem Soc. 2011; 133(39):15721-9.
Microfluidics using spatially defined arrays of droplets in one, two, and three dimensions. Pompano RR, Liu W, Du W, Ismagilov RF. Annu Rev Anal Chem. 2011; 4:59-81. (review)
Microfluidics for analysis of complex biological systems:
Confinement regulates complex biochemical networks: initiation of blood clotting by “diffusion acting”. Shen F, Pompano RR, Kastrup CJ, Ismagilov RF. Biophys J. 2009 Oct 21;97(8):2137-45.
Spatial localization of bacteria controls coagulation of human blood by ‘quorum acting’. Kastrup CJ, Boedicker JQ, Pomerantsev AP, Moayeri M, Bian Y, Pompano RR, Kline TR, Sylvestre P, Shen F, Leppla SH, Tang WJ, Ismagilov RF. Nat Chem Biol. 2008; 4(12):742-50.
Rate of mixing controls rate and outcome of autocatalytic processes: theory and microfluidic experiments with chemical reactions and blood coagulation. Pompano RR, Li HW, Ismagilov RF. Biophys J. 2008; 95(3):1531-43.
Lin Pu
Education
Ph.D. University of California San Diego, 1990
Postdoctoral Fellow, Stanford University, 1991-1992
Postdoctoral Fellow, California Institute of Technology, 1992-1994
Organic, Polymer and Organometallic Chemistry; Asymmetric Catalysis; Chiral Sensors; Optically Active Materials
Multi-disciplinary research programs involving organic synthesis, molecular recognition, fluorescent sensing, asymmetric catalysis, and polymers are conducted in our laboratory. The 1,1′-bi-2-naphthol (BINOL) and its derivatives are chosen as the chiral building blocks to construct novel chiral molecules and macromolecules for diverse applications. We have developed a family of enantioselective fluorescent sensors for the recognition of organic molecules such as alpha-hydroxycarboxylic acids, amino acids, amino alcohols, and amines. These sensors are potentially useful for rapid assay of the enantiomeric composition of chiral compounds and for high throughput chiral catalyst screening. They are also potentially useful for biological analysis and imaging. New chiral conjugated polymers and dendrimers are prepared for applications in materials, catalysis, and sensing. We have discovered that the Lewis acid complexes of the optically active binaphthyl molecules and polymers can carry out highly enantioselective organic reactions such as organozinc additions to aldehydes, hetero-Diels-Alder reactions, 1,3-dipolar cycloadditions, reductions of ketones, Michael additions, epoxidations, and others. Interesting chiral organic molecules including those of biological functions are prepared by using these catalysts.
Enantioselective Fluorescent Sensors
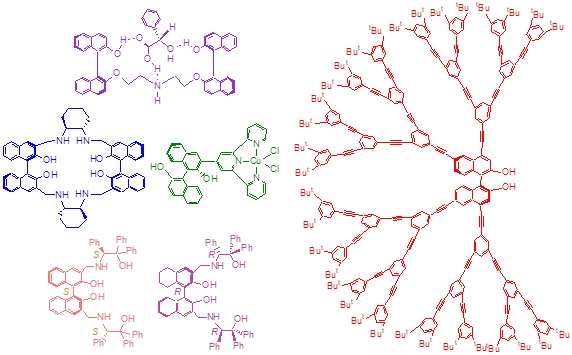
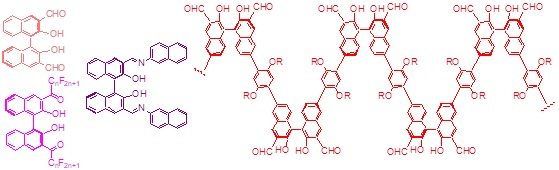
Asymmetric Catalysts
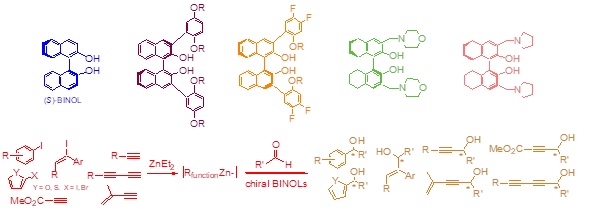
Recent Publications
Enantioselective Fluorescent Recognition of Free Amino Acids: Challenges and Opportunities. Pu, L. Angew. Chem. Int. Ed. 2020, 59, 21814-21828.
Free Amino Acid Recognition: A Bisbinaphthyl-Based Fluorescent Probe with High Enantioselectivity. Zhu, Y.-Y.; Wu, X.-D.; Gu, S.-X.; Pu, L. J. Am. Chem. Soc. 2019, 141, 175−181.
Simultaneous Determination of Concentration and Enantiomeric Composition in Fluorescent Sensing. Lin Pu. Acc. Chem. Res. 2017, 50, 1032-1040.
Regiospecific Hydration of N-(Diphenylphosphinoyl)propargyl amines: Synthesis of b-Amino Ketones by Au(III) Catalysis. Ying, J.; Pu, L. J. Org. Chem. 2016, 81, 8135−8141. A Featured Article. Highlight on the cover.
Conjugated polymer-enhanced enantioselectivity in fluorescent sensing. Zhang, X. –P.; Wang, C.; Wang, P.; Du, J. –J.; Zhang, G. –Q.; Pu, L. Chem. Sci. 2016, 7, 3614–3620.
Rational Design of a Fluorescent Sensor to Simultaneously Determine Both the Enantiomeric Composition and Concentration of Chiral Functional Amines. Wen, K. –L.; Yu, S. –S.; Huang, Z.; Chen, L. –M.; Xiao, M.; Yu, X. –Q.; Pu, L. J. Am. Chem. Soc. 2015, 137, 4517-4524.
Asymmetric Functional Organozinc Additions to Aldehydes Catalyzed by BINOLs. Pu, L. Acc. Chem. Res. 2014, 47, 1523–1535.
Zn(II) Promoted Dramatic Enhancement in the Enantioselective Fluorescent Recognition of Chiral Amines by a Chiral Aldehyde. Huang, Z.; Yu, S. S.; Yu, X. Q.; Pu. L. Chem. Sci. 2014, 5, 3457-3462.
Enantioselective Fluorescent Sensors: A Tale of BINOL. Pu, L. Acc. Chem. Res. 2012, 45, 150–163.
Simultaneous Determination of Both the Enantiomeric Composition and Concentration of a Chiral Substrate with One Fluorescent Sensor. Yu, S.; Plunkett, W.; Kim, M.; Pu, L. J. Am. Chem. Soc. 2012, 134, 20282–20285. A spotlight article reported in J. Am. Chem. Soc., 2013, 135 (3), 949–950.
1,1'-BINAPHTHYL-BASED CHIRAL MATERIALS: OUR JOURNEY


Laura Serbulea
Education
B.S. University of Bucharest, Romania, 1989
Ph.D. University of California, Los Angeles, 2009
Professor Serbulea is teaching organic chemistry courses, including the accelerated organic chemistry lectures and laboratories. She is actively involved in the development of the organic chemistry curriculum, focusing on improving the coordination between the topics in the lecture course and the experiments that are carried out in the laboratory. In the accelerated organic chemistry laboratories, students gain hands-on experience in the synthesis, purification, and characterization of organic compounds using modern analytical instruments and laboratory equipment.
Laura Serbulea earned her undergraduate degree at the University of Bucharest, Romania, and received her Ph.D. in Chemistry from University of California, Los Angeles. She joined the faculty at the University of Virginia in 2012. Her research interests are in the area of computational drug design targeting G-protein coupled receptors, and investigation of organic reaction mechanisms using computational methods.

Diane M. Szaflarski
Education
B.A. University of Minnesota, 1982
Ph.D. University of California, Los Angeles, 1988
My work focuses on considering what Chemical Education should look like today and in future decades. Chemistry, “the central science” has many important pillars that have to be established in order to understand advanced applications and mechanisms. It is essential that as chemical educators we continuously and critically evaluate whether our curricula are current in applications and technologies that are relevant to today’s science and industry, and will be applicable for our students as they encounter their future.
I define Chemical Education broadly and am interested in the range of students including chemistry, science and chemical education majors as well as non-science majors. The focus of the non-majors course that I teach, Chem 1210, is to address college science literacy and to explore the question of what every college student should know about chemistry and impact of chemical issues. In my courses, I strive to establish student centered learning environments in the classroom to maximize the student involvement and focus.
My consideration of curricula parallels my interest in building support networks, especially for those students taking college level chemistry for the first time. It is important that the appropriate supports are in place so that student’s interest does not wane as a result of frustration. I have been involved in departmental efforts to establish support for students.
I am interested in bridging the Chemistry Department with other areas on campus, and have done this with the Curry School and the School of Nursing. These connections encourage facile flow of information on teaching methods and current chemistry content which allows us superior approaches to serving the needs of today’s students. I currently teach in the Chemistry Department and the School of Nursing.

B. Jill Venton
Education
B.S. University of Delaware, 1998
Ph.D. University of North Carolina, 2002
NIH Postdoctoral Fellow, University of Michigan, 2003-2005
The Venton group is interested in the development and characterization of analytical techniques to measure neurochemical changes. Measurements in the brain are challenging because tiny quantities of neuroactive molecules must be detected in a chemically-complex sample while disturbing the tissue as little as possible. In addition, fast time resolution measurements are needed to track the fast dynamics of neurotransmitter release and uptake. Our lab develops both electrochemical and separations methods to monitor these rapid changes in neurotransmitters in model systems. The development of new analytical tools will enable a better understanding of the central nervous system and facilitate the development of new treatments for neurological disorders. Several specific projects are highlighted below:
Electrochemical Detection of Adenosine
Adenosine is a neuromodulator that has a variety of actions including regulation of cerebral blood flow, modulation of neurotransmission, and protection against neuronal injury during stroke. We are studying the regulation of adenosine release in vivo and changes in adenosine in a brain slice model of stroke using cyclic voltammetry at carbon-fiber microelectrodes. Recently, we started to expand this study of adenosine dynamics to understand whether there are any differences between males and females.
Detection of Neurotransmitter Release in Drosophila
Drosophila melanogaster (the fruit fly) is a favorite model organism for biologists, but the central nervous system of a Drosophila larva is only 8 nL in volume! Our main tool is implanting a microelectrode that can detect rapid neurotransmitter changes. A secondary technique we have developed is capillary electrophoresis with fast-scan cyclic voltammetry detection for investigating tissue content. We are currently developing methods to study both serotonin and dopamine release in the fly. While initial methods were developed in the larvae, we are now studying the adult as well, targeting the central complex and the mushroom bodies. These studies are fast and will allow access to a wide array of genetics that are possible in the fly. Current investigations include models of Parkinson disease in the fly and how autoreceptor drugs function on the serotonin system.
Development of Carbon Nanomaterial-Based Electrodes
Carbon nanotubes have interesting electrical, chemical and mechanical properties and have been shown to promote electron transfer in electrochemical experiments. The Venton Lab explores the electrochemical properties of carbon nanomaterial-modified and carbon nanostructured electrodes to improve the sensitivity, temporal resolution, and antifouling properties for the FSCV detection of neurotransmitters. Recently, we have characterized carbon nanospikes, carbon nanohorns, and carbon nanopipette electrodes. We are also 3D printing carbon electrodes with custom geometries.
Recent Publications
Real-Time Measurement of Stimulated Dopamine Release in Compartments of the Adult Drosophila melanogaster Mushroom Body. M. Shin, J. M. Copeland, B. J. Venton. Analytical Chemistry, 92(21): 14398-14407 (2020).
A1 and A2A Receptors Modulate Spontaneous Adenosine but Not Mechanically Stimulated Adensoine in the Caudate. Y. Chang, Y. Wang, B. J. Venton. ACS Chemical Neuroscience, 11(20): 3377-3385 (2020).
Improving Serotonin Fast-Scan Cyclic Voltammetry Detection: New Waveforms to Reduce Electrode Fouling. K. E. Dunham, B. J. Venton. Analyst, 145: 7437-7446 (2020).
Thin Layer Cell Behavior of CNT Yarn and Cavity Carbon Nanopipette Electrodes: Effect on Catecholamine Detection. Z. Shao, P. Puthongkham, K. Hu, R. Jia, M. V. Mirkin, B. J. Venton. Electrochimica Acta, 361(20): 137032 (2020).
3D-Printed Carbon Nanoelectrodes for In Vivo Neurotransmitter Sensing. Q. Cao, M. Shin, N. V. Lavrik, B. J. Venton. Nano Letters, 20(9): 6831-6836 (2020).
Structural Similarity Image Analysis for Detection of Adenosine and Dopamine in Fast-Scan Cyclic Voltammetry Color Plots. P. Puthongkham, J. Rocha, J. R. Borgus, M. Ganesana, Y. Wang, Y. Chang, A. Gahlmann, B. J. Venton. Analytical Chemistry, 92(15): 10485-10494 (2020).
Optimization of Graphene Oxide-Modified Carbon-Fiber Microelectrode for Dopamine Detection. Y. Chang, B. J. Venton. Analytical Methods, 12: 2893-2902 (2020).
Complex Sex and Estrous Cycle Differences in Spontaneous Transient Adenosine. J. R. Borgus, P. Puthongkham, B. J. Venton. Journal of Neurochemistry, 153(2): 216-229 (2020).
Recent Advances in Fast-Scan Cyclic Voltammetry. P. Puthongkham, B. J. Venton. Analyst, 145: 1087-1102 (2020).
Fundamentals of Fast-Scan Cyclic Voltammetry for Dopamine Detection. B. J. Venton, Q. Cao. Analyst, 145: 1158-1168 (2020).
Awards and Honors
- Professor Venton was named the Distinguished Research of the Year by the ACS Virginia Section in January 2020
- Professor Venton’s “Review: Carbon nanotube based electrochemical sensors for biomolecules” is top cited review
- Society for Electroanalytical Chemistry Young Investigator Award, 2011
- Camille Dreyfus Teacher-Scholar, 2010
- American Chemical Society PROGRESS/Dreyfus Foundation Lectureship, 2008
Kevin Welch
Education
B.Sc. Gettysburg College, 2002
Ph.D. University of Virginia, 2007
Professor Kevin Welch is interested in developing curricula for undergraduate instruction in general chemistry and organic chemistry. In particular, his focus is on updating these courses to accommodate the diverse educational background in chemistry of the students enrolling in chemistry at the University of Virginia, as well as providing a strong chemical foundation for the students as they continue on in their educational and post-educational careers in a variety of fields. In the past, he has taught undergraduate courses in general chemistry, organic chemistry, inorganic chemistry, environmental chemistry, and scientific writing.
In addition to a focus on undergraduate education, Kevin’s interests in chemistry involve research investigating metal-ligand bonding interactions and the use of transition metal complexes to address challenges in fuel generation and energy storage.
A native of northern Virginia, Kevin received his B.S. in chemistry from Gettysburg College in 2002, and his Ph.D. in chemistry from the University of Virginia in 2007, where he focused on synthetic organometallic chemistry. He spent three years in central Washington as a Department of Energy Postdoctoral Researcher at the Pacific Northwest National Laboratory, working on the development of transition metal catalysts for energy storage and fuel cell technology. From 2010 to 2016, Kevin was a visiting assistant professor at Swarthmore College outside of Philadelphia, Pennsylvania, where he taught and conducted research with undergraduate students. He returned to the University of Virginia in the fall of 2016 and currently teaches courses in general chemistry and laboratories for organic chemistry.

Lindsay Wheeler
Education
B.S. Virginia Commonwealth University, 2004
M.A. University of Virginia, 2006
M.T. University of Virginia, 2008
Ph.D. University of Virginia, 2015
Professor Lindsay Wheeler is a lecturer in the Chemistry Department and Assistant Director of STEM education innovations in the Center for Teaching Excellence (CTE). Dr. Wheeler teaches STEM education and higher education courses in the Education School. She previously taught a 1-credit Teaching Methods in Higher Education for chemistry, physics, biology, and astronomy TAs, which will be transformed into asynchronous modular units for TAs to access and integrate into their teaching. She worked with Dr. Charles Grisham to redesign the General Chemistry laboratory curricula (Chem 1411/1611/1421/1621) to a project-based guided inquiry approach where students work collaboratively to design, implement, analyze, and communicate their approach to solving a real-world problem. Dr. Wheeler has also previously taught Chem 1400 using a Process Oriented Guided Inquiry Learning (POGIL) curriculum.
Dr. Wheeler’s mixed methods research explores the impact of professional development programs and teaching interventions on instructors’ beliefs, confidence, and practice in undergraduate STEM classrooms. She also investigates the impact of TA training and Teaching Methods courses on TAs’ content knowledge, beliefs, confidence, and instructional practice as well as how these TAs influence the students they teach. Dr. Wheeler is also the PI of a research collaborative across 18 institutions to capture the experiences of educational developers, faculty, and academic leadership during the time of crises.
Dr. Wheeler is active in the science education and educational development professional communities and presents regularly at national and international conferences. She is Associate Coeditor of To Improve the Academy: A Journal of Academic Development. She leads the SoTL Scholars program to empower and enable faculty to engage in classroom-based research and disseminate their findings.
Recent publications:
Cruz, L., Dickens, E., Flaming, A., & Wheeler, L. (Accepted). Under the (compound) microscope: Multiple perspectives on the scholarship of educational development. International Journal of Academic Development.
Favre, D., Bach, D., & Wheeler, L.B. (Accepted). Measuring institutional transformation: A multi-faceted assessment of a new faculty development program. Journal of Research in Innovation Teaching & Learning.
St. Claire, T., Wheeler, L.B., Maeng, J.L., & Bell, R.L. (2020). Mixed-Methods Analysis of Science Teacher Educator Professional Development. Professional Development in Education. DOI: 10.1080/19415257.2020.1787191
Wheeler, L. & Bach, D. (2020). Understanding the Impact of Educational Development Interventions on Classroom Instruction and Student Success. International Journal of Academic Development. DOI:10.1080/1360144X.2020.1777555
Wheeler, L., Palmer, M., & Aneece, I. (2019). Students’ perceptions of course syllabi: The role of syllabi in motivating students. International Journal of the Scholarship of Teaching and Learning, 13(3), 1-10.
Sturtevant, H. & Wheeler, L. (2019). The STEM Faculty Instructional Barriers and Identity Survey (FIBIS): Development and exploratory results. International Journal of STEM Education, 6(35), 1-22. DOI: 10.1186/s40594-019-0185-0
- Featured on Tea for teaching
- Featured in the Chronicle of Higher Education
Wheeler, L., Sturtevant, H., & Mumba, F. (2019). An exploratory study of the impact of a Teaching Methods course for International Teaching Assistants in an inquiry-based general chemistry laboratory. Journal of Chemical Education. DOI: 10.1021/acs.jchemed.9b00239
Wheeler, L.B, Mulvey, B.K., Maeng, J.L., Librea-Carden, M.R., & Bell, R. L. (2019). Teaching the Teacher: Exploring STEM Graduate Students’ Nature of Science Conceptions in a Teaching Methods Course. International Journal of Science Education, 41, 1905-1925. DOI: 10.1080/09500693.2019.1647473
Wheeler, L. B., Navy, S. L., Maeng, J.L., & Whitworth, B.A. (2019). Development and Validation of the Classroom Observation Protocol for Engineering Design (COPED). Journal of Research in Science Teaching, 56, 1285-1305. DOI: 10.1002/tea.21557
Wheeler, L.B., Chiu, J.L., Maeng, J.L., & Bell, R.L (2018). Teaching assistant motivation: Learning to teach in an inquiry-based undergraduate laboratory context. Chemical Education Research and Practice. DOI: 10.1039/c8rp00157j.
Stains, M., Harshman, J., Barker, M.K., Chasteen, S.V., Cole, R., DeChenne-Peters, S.E., Eagan Jr., M.K., Esson, J.M., Knight, J.K., Laski, F.A., Levis-Fitzgerald, M., Lee, C.J., Lo, S.M., McDonnell, L.M., McKay, T.A., Michelotti, N., Musgrove, A., Palmer, M.S., Plank, K.M., Rodela, T.M., Sanders, E.R., Schimpf, N.G., Schulte, P.M., Smith, M.K., Stetzer, M., Van Valkenburgh, B., Vinson, E., Weir, L.K., Wendel, P.J., Wheeler, L.B., Young, A.M. (2018). Anatomy of STEM teaching in American universities: A snapshot from a large-scale observation study. Science, 359, 1468-1470. DOI: 10.1126/science.aap8892
Wheeler, L.B., Maeng, J.L., Chiu, J.L., & Bell, R.L. (2017). Do teaching assistants matter? Investigating relationships between teaching assistants and student outcomes in undergraduate science laboratory classes. Journal of Research in Science Teaching, 54, 463-492. DOI: 10.1002/tea.21373
Wheeler, L.B., Clark, C.P., & Grisham, C.M. (2017). Transforming a traditional laboratory to an inquiry-based course: Importance of training TAs when redesigning a curriculum. Journal of Chemical Education, 94, 1019-1026. http://dx.doi.org/10.1021/acs.jchemed.6b00831
Wheeler, L.B., Maeng, J.L., & Whitworth, B.A. (2017). Teaching assistant (TA) professional development within an inquiry-based general chemistry context: Characterization of TA knowledge and beliefs. Journal of Chemical Education, 94, 19-28. DOI: 10.1021/acs.jchemed.6b00373
Palmer, M.P., Wheeler, L.B., & Aneece, I.P. (2016). Does the document matter? The evolving role of syllabi in higher education, Change: The Magazine of Higher Learning, 48 (4), 36-47. DOI: 10.1080/00091383.2016.1198186
Wheeler, L.B., Chiu, J.L., & Grisham, C.M. (2016). Computational methods in general chemistry: Perceptions of programming, prior experience, and student outcomes. Journal of College Science Teaching, 45, 3, 83-91.
Wheeler, L.B., Maeng, J.L., & Whitworth, B.A. (2015). Teaching assistants perceptions of a training to support an inquiry-based general chemistry laboratory course. Chemical Education Research and Practice, 16, 824-842. DOI: 10.1039/c5rp00104h

Sen Zhang
Education
B.S. University of Science and Technology of China, 2008
Ph.D. Brown University, 2013
NatureNet Postoctoral Fellow, University of Pennsylvania, 2013-2016
The Zhang group focuses on the development of inorganic nanoscale and sub-nanoscale materials with atomically precise surfaces, interfaces and ordered architectures that enables notable physicochemical properties and emerging applications. Combining the efforts in materials synthesis, advanced characterization, and functional device engineering and simulation, we aim to derive new design principle and new synthetic methodology for critical materials in catalysis, magnetism, plasmonics and biosensing.
Synthetic Methodology for Atomically Precise Materials: Central to functionalizing materials is our ability to synthetically control materials physical dimensions, compositions, and structures with atomic precision. We concentrate our research on the fundamental aspects of inorganic solid nucleation and formation mechanisms. Using colloidal chemistry and operando X-ray scattering and spectroscopic probes, we seek to elucidate the reaction kinetics governing the transformation from atomic monomer to nanocrystals with atomically well-defined structures and surfaces. In particular, we are interested in the nonclassical nucleation and growth phenomena mediated by magic-sized clusters and mesocrystals, and the design of new functional ligands that allow the surface atomic structure engineering of nanocrystals and the programmable formation of ordered nanocrystal superlattices.
Catalysis for Clean Energy and Environmental Sustainability: Taking advantage of synthetic control over atomic surfaces and sites of catalytic materials, our research contributes to the fundamental understanding of structure-property relationship of nano/single-site catalysts for critical energy conversion and chemical transformation processes. Ongoing projects include the development of electrocatalysts for hydrogen economy (water electrolyzer and low-temperature fuel cells) and sustainable carbon management (CO2 reduction and biomass-derived molecule conversion) as well as the thermal catalysts for CO2 hydrogenation and light alkane dehydrogenation. A suite of operando spectroscopic tools (X-ray absorption spectroscopy and X-ray photoelectron spectroscopy), in combination with synchrotron radiation X-ray diffraction and environmental electron microscopy, are employed in our research to reveal the atomic structure of catalysts and their evolution under reaction conditions. Our in-house surface enhanced infrared absorption spectroscopy (SEIRAS) and differential electrochemical mass spectrometry (DEMS) allow us to elucidate the reaction pathways and kinetics. This mechanistic investigation over atomically precise material can be seamlessly integrated with theoretical computation for an ultimate understanding of catalyst design and optimization.
Functional Magnetic and Plasmonic Materials: Another area of focus for the Zhang group is the dimension-dependent magnetic and plasmonic properties of nanomaterials. We are interested in the modulation of magnetic anisotropy using 1-D and 2-D metal oxide materials and their ordered assemblies. In addition, we are collaborating with bioanalytic chemists and engineers to design new strategies to modify biological objects (cells, bacteria, viruses, etc.) with functionalized plasmonic and magnetic nanocrystals to enable the efficient sensing in microfluidic devices.
Recent publications
Chang Liu, Jin Qian, Yifan Ye, Hua Zhou, Cheng-Jun Sun, Colton Sheehan, Zhiyong Zhang, Gang Wan, Yi-Sheng Liu, Jinghua Guo, Shuang Li, Hyeyoung Shin, Sooyeon Hwang, T. Brent Gunnoe, William A. Goddard III*, Sen Zhang*, “Oxygen Evolution Reaction over Catalytic Single-Site Co in a Well-Defined Brookite TiO2 Nanorod Surface”, Nature Catalysis 2021, 4, 36-45.
Perrin Godbold, Grayson Johnson, Akachukwu D. Obi, Rebecca Brown, Sooyeon Hwang, Robert J. Gilliard Jr.*, Sen Zhang*, “Surfactant Removal for Colloidal Nanocrystal Catalysts Mediated by N-Heterocyclic Carbenes” J. Am. Chem. Soc. 2021, 143, 2644–2648.
Yulu Zhang, Na Li, Zhiyong Zhang, Shuang Li, Meiyang Cui, Lu Ma, Hua Zhou, Dong Su, and Sen Zhang* "Programmable Synthesis of Multimetallic Phosphide Nanorods Mediated by Core/Shell Structure Formation and Conversion" J. Am. Chem. Soc., 2020, 142, 8490-8497.
Cheng Hao Wu, Chang Liu, Dong Su, Huolin L. Xin, Hai-Tao Fang, Baran Eren, Sen Zhang*, Christopher B. Murray*, Miquel B. Salmeron*, "Bimetallic Synergy in Cobalt-Palladium Nanocatalysts for CO oxidation", Nature Catalysis, 2019, 2, 78-85.
Zhiyong Zhang, Qiyuan Wu, Grayson Johnson, Yifan Ye, Xing Li, Na Li, Meiyang Cui, Jennifer D. Lee, Chang Liu, Shen Zhao, Shuang Li, Alexander Orlov, Christopher B. Murray, Xu Zhang, T. Brent Gunnoe, Dong Su*, and Sen Zhang*. "Generalized Synthetic Strategy for Transition Metal Doped Brookite-Phase TiO2 Nanorods" J. Am. Chem. Soc., 2019, 141, 16548-16552.
Li An, Zhiyong Zhang, Jianrui Feng, Fan Lv, Yuxuan Li, Rui Wang, Min Lu, Ram B. Gupta, Pinxian Xi*, Sen Zhang*, "Heterostructure-Promoted Oxygen Electrocatalysis Enables Rechargeable Zinc-Air Battery with Neutral Aqueous Electrolyte", J. Am. Chem. Soc., 2018, 140,17624-17631.
Chang Liu, Zhong Ma, Meiyang Cui, Zhiyong Zhang, Xu Zhang, Dong Su, Christopher B. Murray, Jia X. Wang, Sen Zhang*, "Favorable Core/Shell Interface within Co2P/Pt Nanorods for Oxygen Reduction Electrocatalysis", Nano Lett., 2018, 18, 7870-7875.
Awards and Honors
2022 NSF CAREER Award, US National Science Foundation
2021 Research Excellence Award, University of Virginia
2021 CAPA Distinguished Junior Faculty Award, Chinese-American Chemistry & Chemical Biology Professors Association (CAPA)
2021 Scialog Collaborative Innovation Award for Negative Emissions Science, Research Corporation for Science Advancement and Alfred P. Sloan Foundation
2020 Rising Star 2020, Frontiers in Chemistry, Frontiers
2020 Emerging Investigator, Nanoscale, The Royal Society of Chemistry
2019 Emerging Investigator, Journal of Materials Chemistry A, The Royal Society of Chemistry
2019 Sustainability Course Development Fellowship, University of Virginia
2014 William R. Potter Prize for Doctoral Thesis of Outstanding Merit, Brown University
2013-2016 NatureNet Postdoctoral Fellowship, The Nature Conservancy
Ralph O. Allen
Education
B.A. Cornell College (Iowa), 1965
Ph.D. University of Wisconsin, 1970
Trace Analysis: Environmental, Archaeological, and Forensic Applications
Professor Allen is not currently accepting graduate students.
The development of sensitive analytical methods has helped provide more detailed information about the small chemical differences between materials which seem similar but have different histories. In some cases, like geological samples, the large scale geochemical processes can give rise to subtle yet understandable differences in those elements which are present at trace levels. We have used instrumental neutron activation analysis and X-ray fluorescence to study several types of geological materials. These materials have been investigated as part of an ongoing effort to interpret the migration of trace elements during geochemical processes. In some cases, these same geological materials have been used by prehistoric humans and hence our studies have had archaeological implications.
To provide accurate trace element data on large numbers of samples, we have investigated several other analytical methods. The inductively coupled plasma emission source used to provide ions for a quadrupole mass spectrometer (ICP-MS) holds considerable promise. This method is also being used to study soil and glass samples for applicability in the forensic laboratory. Our efforts in the forensic science field are part of a continuing cooperative effort with scientists at the FBI’s research facilities. In the past, traces of explosive residues and epoxies have been analyzed to differentiate the sources of the materials. The latest forensic science efforts have been directed toward the important new area of DNA “fingerprinting.” This major forensic tool allows small amounts of genetic material to be used to match the DNA from a suspect.
Representative Publications
Forensic determination of ricin and the alkaloid marker ricinine from castor bean extracts. Darby SM, Miller ML, Allen RO. J Forensic Sci. 6, 1033-42 (2001).
A mass spectrometric method for quantitation of intact insulin in blood samples. Darby SM, Miller ML, Allen RO, LeBeau M. J Anal Toxicol. 25, 8-14 (2001).
Analysis of two multiplexed short tandem repeat systems using capillary electrophoresis with multiwavelength fluorescence detection. Isenberg AR, Allen RO, Keys KM, Smerick JB, Budowle B, McCord BR. Electrophoresis. 19, 94-100 1998 .
Lester S. Andrews
Education
B.S. Mississippi State University
Ph.D. University of California, Berkeley
Spectroscopy and Photochemistry of Matrix Isolated Species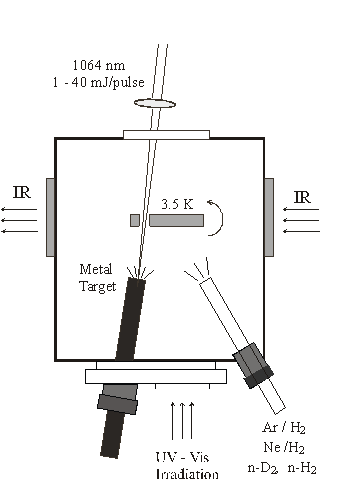
Normally inaccessible chemical species including free radicals, hydrogen-bonded complexes, unusual inorganic species, transition metal containing intermediates, metal hydrides, and molecular ions are under investigation using infrared matrix-isolation spectroscopy. These transient molecules are prepared by reactions of suitable atoms and/or molecules prediluted in argon by precursor photolysis, or by microwave discharge of the reagents during condensation at 7 K in a vacuum chamber. Solid argon isolates the reaction or photolysis products and preserves them for spectroscopic study at 7 K. Neon is used to isolate new reactionproducts on a 4 K substrate. Pure H2 and D2 are also be employed as a reagent and matrix for the investigation of novel metal hydrides. A new source of atoms that require high temperatures to generate, namely pulsed laser ablation from solid samples, has been developed for use in matrix-infrared spectroscopy in this laboratory. This method exploits two advantages: first, the ablated atoms contain excess energy which can activate reactions with small molecules, and second, collisions with matrix atoms during the condensation process relax energetic product molecules and allow them to be trapped in the solid matrix for spectroscopic study. In addition, laser-ablation also produces cations and electrons for reactions to make charged products.
The experimental apparatus used in hydrogen matrix investigations is sketched in the figure. Infrared spectra are recorded after co-depositing reagent and host matrix gases on the cold sample window and rotating by 90 degrees to face the infrared light beam.
These infrared spectroscopic matrix-isolation studies characterize the bonding and structure of chemical intermediates, interesting new inorganic molecules and complexes and molecular ions and often provide a useful complement and guide to high resolution gas phase work. Stable isotopes are used to determine assignments of the observed infrared absorptions to fundamental vibrational frequencies. Comparison of vibrational energies within related chemical species provides conclusions about the bonding of these newly observed chemical intermediates. Selection rules based upon molecular symmetry and vibrational analysis help determine the molecular geometry. Ab initio electronic structure calculations are done to find molecular structures compatible with the infrared spectrum. Agreement between calculated and observed isotopic vibrational spectra provides further evidence for the discovery of new transient molecular species.
Please click here for a full list of publications.
Awards and Honors

Robert F. Bryan
Education
B.S. University of Glasgow, Scotland
Ph.D. University of Glasgow, Scotland
Robert Bryan was a faculty member of the U.Va. Chemistry Department for 36 years. During his career, Professor Bryan established an international reputation in crystallography and published more than 150 papers in the area. He was born in Rhu, Scotland in 1933, and earned his B.Sc. (1954) and Ph.D. (1957) from the University of Glasgow. His postdoctoral training was at ETH (BAttelle Fellow) and MIT (Alfred P. Sloane Fellow and a NIH Fellow) from 1957 – 1961. In 1961, he joined the faculty at Johns Hopkins University School of Medicine in the Biophysics Department. He joined U.Va. in 1967 as an Associate Professor of Chemistry and became a Full Professor in 1982. He held several offices in the international Union of Crystallography and was an editor of Acta Crystallographica and The Journal of Applied Crystallography. In addition to a very active research career (over 150 publications), Prof. Bryan was the Associate Dean for the Graduate School of Arts and Sciences from 1970 – 1973.

Robert G. Bryant
Education
A.B. Colgate University, 1965
Ph.D. Stanford University, 1969
Nuclear Magnetic Resonance in Chemistry
The Bryant laboratory utilizes a wide range of nuclear and electron spin resonance methods to characterize intra and intermolecular dynamics. A primary focus is the rich information available from the study of the magnetic field dependence of the nuclear spin-lattice relaxation rate as a function of the magnetic field strength or what is called the magnetic relaxation dispersion (MRD). We have constructed unique instrumentation that permits acquisition of MRD profiles for a variety of nuclear resonances in high resolution. These measurements provide a direct measure of the time fluctuations in various nuclear or nuclear-electron dipolar couplings. The resulting MRD profile reports relative inter and intramolecular motions over the time range from about ten microseconds to one picosecond. We are developing methods for probing the fluctuation spectrum of specific vectors defined in structurally interesting proteins and are trying to understand how these fluctuations affect molecular function. In related work, we apply the nuclear spin relaxation induced by freely diffusing paramagnetic solutes in cosolutes to define how one molecule samples the local environment of another and explores the surface or even penetrates into a complex structure like a folded protein. The measurements are sensitive to relatively weak intermolecular interactions and permit definition of highly localized intermolecular free energy differences in solutions. Multinuclear studies of proteins in a number of dynamical environments provide a fundamental characterization of how the protein structure fluctuates in time and how energy is redistributed in the folded structure. The practical implications range from understanding protein catalytic function to developing new techniques for diagnostic medicine in the context of magnetic resonance imaging or MRI.
Recent Publications
Water-proton-spin-lattice-relaxation dispersion of paramagnetic protein solutions. Diakova G, Goddard Y, Korb JP, Bryant RG. J Magn Reson. 208:195-203 (2010).
Water and backbone dynamics in a hydrated protein. Diakova G, Goddard YA, Korb JP, Bryant RG. Biophys J. 98:138-46 (2010).
Dynamics of water in and around proteins characterized by 1H Spin-Lattice Relaxometry. Bryant RG. Comptes Rendus Physique. 11(2), 128-135(2010).
Dimensionality of diffusive exploration at the protein interface in solution. Grebenkov DS, Goddard YA, Diakova G, Korb JP, Bryant RG. J Phys Chem B. 113, 13347-13356 (2009).
Water molecule contributions to proton spin-lattice relaxation in rotationally immobilized proteins. Goddard YA, Korb JP, Bryant RG. J Magn Reson. 199, 68-74 (2009).
Robert Burnett

Francis A. Carey
Education
B.S. Drexel University
Ph.D. Penn State
Francis A. Carey is a native of Pennsylvania, educated in the public schools of Philadelphia, at Drexel University (B.S. in chemistry, 1959), and at Penn State (Ph.D. 1963). Following postdoctoral work at Harvard and military service, he was appointed to the chemistry faculty of the University of Virginia in 1966. Prior to retiring in 2000, he regularly taught the two-semester lecture courses in general chemistry and organic chemistry. With his students, Professor Carey has published over forty research papers in synthetic and mechanistic organic chemistry. In addition to this text, he is coauthor (with Robert C. Atkins) of Organic Chemistry: A Brief Course and (with Richard J. Sundberg) of Advanced Organic Chemistry, a two-volume treatment designed for graduate students and advanced undergraduates. He was a member of the Committee of Examiners of The Graduate Record Examination in Chemistry from 1993-2000.

Graeme Gerrans
Education
B.S. Rhodes University, South Africa
Ph.D. Cambridge University, England
Professor Gerrans is primarily engaged in teaching and chemical education. He has taught undergraduate chemistry at the University of the Witwatersrand, South Africa and here at the University of Virginia. He has held visiting professorships at the California Institute of Technology (1972), the University of Illinois (1979), the University of East Anglia, England (1986) and the University of Virginia (1990). Amongst the awards that he has received are the Convocation Distinguished Teacher’s Award (University of the Witwatersrand) and the Education Medal (South African Chemical Institute). He has published over 80 papers and is the co-author, together with P and R Hartmann-Petersen, of “Encyclopedia of Science and Technology,” (New Africa Books, 2007).
Most recently, Professor Gerrans was a faculty in the Spring 2009 UVA Semester at Sea Program.

Russell N. Grimes
Education
B.S. Lafayette College
Ph.D. University of Minnesota
Russ Grimes was raised in Pennsylvania and is a B.S. Chemistry alumnus of Lafayette College He received his Ph.D. in Chemistry from the University of Minnesota as a student of Professor William Lipscomb, although his thesis research was conducted at Harvard University where he was a Harvard Fellow. Following postdoctoral work at Harvard and with Professor M. F. Hawthorne at the University of California, Riverside, he was a faculty member in the Department of Chemistry from 1963 to 2003, becoming a Full Professor in 1973 and serving a term as Department Chair from 1981 to 1984. Over a 40-year period he and his students were among the pioneers in the synthesis and study of polyhedral molecular cage compounds of boron, along the way discovering metal-promoted oxidative cage fusion, tricarbon and large tetracarbon carboranes, stable triple-decker sandwich complexes (which were also the first electrically neutral triple-deckers), isolable tetra-, penta-, and hexadecker molecular sandwiches, linked multidecker complexes, polyhedral metallaboranes, and many novel clusters ranging from C2B3H7 to 14-vertex M2C4B8 systems. A major theme in their work was the development and exploration of small carborane ligands in the designed synthesis of novel transition-metal organometallic systems, especially those having electrically conducting or catalytic properties. The Grimes group together with William Hutton, in work initially reported in the Journal of the American Chemical Society in 1980 and 1981, developed the first successful 2D COSY (correlated spectroscopy) NMR technique involving a quadrupolar nucleus (11B) and in a 1984 JACS full paper detailed its use in both homonuclear 11B-11B) and heteronuclear (11B-1H) spectroscopy.
Professor Grimes was a Senior Fulbright Scholar at the University of Canterbury, New Zealand, in 1974-75, a Guest Professor at the University of Heidelberg, Germany, in 1986, a Humboldt Scholar there in 1997-98, and a Visiting Scholar at the Korea Advanced Institute for Science and Technology in 1997. He received a Boron U.S.A. (BUSA) Award for Distinguished Achievements in Boron Science in 1990 and was elected a Fellow of the American Association for the Advancement of Science in 1981. He served as an American Chemical Society Tour Speaker on six occasions. He is the author or co-author of 240+ research publications and the books Carboranes (Academic Press, 1970, Russian translation 1974) and Carboranes Second Edition, (Elsevier, 2011). He edited the book Metal Interactions with Boron Clusters (Plenum, 1982) and is Editor-in-Chief of Inorganic Syntheses, Vol. 29 (John Wiley, 1992). He has served on the editorial board of Inorganic Chemistry and the editorial review panel of Dalton Transactions, and is a past president of the Board of Directors of Inorganic Syntheses.
The Third Edition of Carboranes was published by Elsevier in August, just 5 years following the Second Edition. The book reflects the rapid pace of development in this field, which is generating hundreds of publications each year in areas as diverse as nanomaterials, pharmacology, diagnostic and therapeutic medicine, extraction of radioactive metals from nuclear waste, catalysis, and organic synthesis. Professor Grimes was recently honored by a special issue of the Journal of Organometallic Chemistry, featuring over 40 contributed papers from international scientists in recognition of his contributions to boron chemistry throughout his 50-year career.

Sidney Hecht
Education
B.A. University of Rochester
Ph.D. University of Illinois
Sidney Hecht was the John W. Mallet Professor of Chemistry and a Professor of Biology at UVA from 1978 – 2008. During his thirty years he led a very productive research group in the areas of synthesis and mechanism of action of bleomycin family antitumor antibiotics, peptidyltransferase as a source of synthetic enzymes, mechanism of action/inhibition of mammalian DNA topoisomerase I, and inhibition of signal transduction at the level of p90RSK. He is the recipient of numerous awards icluding the Alfred P. Sloan Research Fellowship (1975-79), a National Institutes of Health Research Career Development Award (1975-80), a John Simon Guggenheim Memorial Fellowship (1977-78), the 1996 Arthur C. Cope Scholar Award, the Virginia’s Outstanding Scientist Award (1996), a 1998 Research Achievement Award, American Society of Pharmacognosy and in 2004 he was elected as a Fellow of the American Association for the Advancement of Science. In a career spanning more than three decades, Sidney Hecht has held both academic and industrial research positions. From 1981 to 1986 in addition to his professorship at UVA, he held concurrent appointments at Smith Kline & French Laboratories, first as Vice President Preclinical R&D, then Vice President Chemical R&D. He is currently Director of the Center for BioEnergetics in the Biodesign Institute and Professor of Chemistry at Arizona State University.
James A. Marshall
Education
B.S. University of Wisconsin (Madison), 1957
Ph.D. University of Michigan, 1960
N.I.H. Postdoctoral Fellow, Stanford University, 1960-1962
In our research we identify natural products with interesting, often novel, structures and useful biological properties that may lead to medicinal agents for treatment of cancer, cardiovascular disorders and infectious diseases. We focus on key structural and stereochemical features present in these target natural products and devise mechanism or analogy based methodology for efficient construction of potentially useful subunits. Finally, we assemble the foregoing subunits by a combination of new and known reactions to synthesize the target substance and/or analogues thereof. Our overall program is thus target directed but methods driven.
Recent Publications
A cascade cyclization route to adjacent bistetrahydrofurans from chiral triepoxyfarnesyl bromides. Marshall JA, Hann RK. J Org Chem. 73, 6753-7(2008).
A formal synthesis of the callipeltoside aglycone. Marshall JA, Eidam PM. Org Lett. 10, 93-6 (2008).
Chiral allylic and allenic metal reagents for organic synthesis. Marshall JA. J Org Chem. 72, 8153-66 (2007).
ABC synthesis and antitumor activity of a series of Annonaceous acetogenin analogs with a threo, trans, threo, trans, threo-bis-tetrahydrofuran core unit. Marshall JA, Sabatini JJ, Valeriote F. Bioorg Med Chem Lett. 17, 2434-7 (2007).
Palladium- and copper-catalyzed 1,4-additions of organozinc compounds to conjugated aldehydes. Marshall JA, Herold M, Eidam HS, Eidam P. Org Lett. 8, 5505-8 (2006).
Synthesis of a bistetrahydrofuran C17-C32 fragment of the polyether antibiotic ionomycin. Marshall JA, Mikowski AM. Org Lett. 8, 4375-8 (2006).

R. Bruce Martin
Education
B.S. Northwestern University
Ph.D. University of Rochester
Bruce received his B.S. degree in chemistry at Northwestern University and received a Ph.D. degree in photochemistry from the University of Rochester. Following postdoctoral appointments at both the California Institute of Technology and Harvard University, Bruce taught briefly at the American University of Beirut in Lebanon and came to the University of Virginia in 1959. Throughout his research career, Professor Martin applied the methodologies of a physical chemist to investigate a wide range of problems of biological inspiration and consequence. When he retired in 1994, he had published more than 200 papers ranging across physical, inorganic, biophysical, bioinorganic, physical organic, and bioorganic chemistry. In 1964, he published a very highly regarded textbook, “Introduction to Biophysical Chemistry” that was translated into multiple languages. Bruce served as Chair during a difficult period in the Department’s history and was the principal catalyst in our transition to a modern Chemistry Department. Bruce’s Chemistry colleagues remember him as a thoughtful and conscientious mentor and friend who went out of his way to support his colleagues and provide valuable counsel, especially to younger faculty. Professor Martin received the President’s and Visitors’ Research Prize of the University three times, the J. Shelton Horsley Research Award of the Virginia Academy of Science, and recognition as a fellow of the AAAS. Since about 2005, Bruce and his wife had lived in Palo Alto, California. Bruce passed away in May 2018.

Frederick S. Richardson
Education
B.S. Dickinson College
Ph.D. Princeton University
Professor Frederick S. Richardson earned his B.S. degree at Dickinson College summa cum laude in 1961. In 1966, he received his Ph.D. at Princeton University working with Walter Kauzmann. He served in the US Army for two years before starting his NSF Postdoctoral Research Fellowship at the University of California, San Diego. In 1969, he joined the faculty in the Chemistry Department at the University of Virginia. Prof. Richardson served as chair of the department from 1983 – 87 and 1992 – 1997. In 1991, he was appointed a Commonwealth Professor. Prof. Richardson is an exceptional physical chemist and is known for his research, teaching, and mentorship.
He was appointed as a Henry Dreyfus Foundation Teacher-Scholar from 1973-78. He taught undergraduate general, physical, and biophysical chemistry, as well as, graduate courses in all areas of physical chemistry. He research supervised 36 Ph.D. students, 6 M.S. students and 28 postdoctoral fellows.
Prof. Richardson has published over 220 papers in scientific journals or books. His principal research interests are:
- Electronic structure and spectroscopy of molecules and ions in solution and in crystals.
- Natural and magnetic chiroptical properties of molecular systems.
- Intermolecular chiral recognition and discrimination phenomena.
- Spectroscopic probes of metal-ion coordination sites in biomolecular systems.
- Rare-earth chemistry and spectroscopy.
- Theory and measurement of nonlinear optical processes in condensed-phase materials.

Paul N. Schatz
Education
B.S. University of Pennsylvania
Ph.D. Brown University
Paul N. Schatz has published widely in spectroscopic areas ranging from the study of absolute infrared intensities to Magnetic Circular Dichroism (MCD) measurements on matrix isolated species using Synchrotron Radiation in the vacuum ultraviolet, as well as on the theory of mixed valence compounds.
He was a pioneer along with Professor Philip J. Stephens in the development of Magnetic Circular Dichroism (MCD) spectroscopy. This included development of one of the first MCD instruments along with the theoretical interpretation of the novel data produced. These studies started with symmetrical inorganic molecules in solution, then moved on to symmetrical organic molecules, and then to crystals doped with such molecules at pumped liquid helium temperatures (~2.7 K) and as a function of temperature. In later years, matrix isolated species were studied, and pioneering studies on these species were carried out using far ultraviolet frequencies available at the Synchrotron Radiation Center in Stoughton Wisconsin.
Along with Professor S. B. Piepho, a comprehensive book emphasizing the group theoretical aspects of MCD was published in 1983.
In 1978, along with Professors S.B. Piepho and Elmars Krausz, the first rigorous theoretical treatment of the spectroscopy of mixed valence compounds was published. In what later became known as the PKS model, both spectroscopic and dynamic aspects of these compounds were explored in detail.

T. Y. Shen
Education
B.S. National Central University of China
Ph.D. University of Manchester, England
Professor Shen was born in Peking, China in 1924. He graduated from National Central University of China in 1946, and a year later traveled to England to study organic chemistry on an Overseas Scholarship form the Chinese Ministry of Education. In 1950, he came to the United States for a postdoctoral position at Ohio State University, followed by a research associateship at MIT. From MIT, he joined Merck & Co. in 1956. He was appointed to the U.Va. Chemistry Department in 1986 as Commonwealth Professor of Chemistry, and in 1986 he was named the Alfred Burger Professor of Medicinal Chemistry. Professor Shen’s research focused on the development of three antiinflammatory – analgesic drugs (indomethacin, clinoril®, Dolobid®), and potential chemo-therapeutic agents for viral infections, immunolgical, neoplastic, and other metabolic disease. He also synthesized and characterized molecular probes for biomembrane receptors and receptor ligands for targeted drug delivery. Professor Shen served on many editorial boards including the Journal of Medicinal Chemistry. He received many awards throughout his career, including the Rene Descartes Medal, the Galileo Medal of Scientific Achievement, and an Achievement Award from the Chinese Institute of Engineers-USA. He was also a fellow of the AAAS.
Richard J. Sundberg
Education
https://news.virginia.edu/content/memoriam-richard-sundberg-influential-researcher-beloved-teacher-chemistryB.S. State University of Iowa, 1959
Ph.D. University of Minnesota, 1962
NIH Postdoctoral Fellow Stanford University, 1971-72
Richard J. "Dick" Sundberg died on November 1, 2021, in Hickory, N.C., after a period of declining health. A resident of Charlottesville, Virginia, since 1964, he had relocated to North Carolina in May 2021, to be close to daughter Jennifer Sundberg Deal and her family. Born on January 6, 1938, at Sioux Rapids Hospital, Sioux Rapids, Iowa, to Ernest and Rosa (Christensen) Sundberg.
Dick grew up on a farm near Linn Grove, Iowa, the eldest of four surviving children. He attended Linn Grove Consolidated School from kindergarten until graduating as valedictorian of the class of 1955, earning letters and honors in sports and journalism. Dick received his BS degree in Chemistry, with highest distinction, from the University of Iowa in 1959; a distinguished military graduate of the Army ROTC, he received a commission as a 2nd Lieutenant. He completed his PhD in chemistry in August, 1962 at the University of Minnesota, which honored him as a Distinguished Alumnus in 2001.
After serving two years in the U.S. Army Chemical Corps, Dick began his long and successful academic career in 1964 at the University of Virginia, Charlottesville. An excellent teacher and mentor, productive researcher, and skillful administrator, he had a global impact on the field of organic chemistry. He was the author of a number of papers, reviews and monographs in his field, and co-authored the preeminent graduate text in organic chemistry, Advanced Organic Chemistry, revising it through five editions. But his character as much as his scholarship— his "unyielding intellectual honesty in his approach to science"— impacted his students, post docs and colleagues, who found him "rigorous but patient, firm but understanding," with a kind and diligent way of discussing problems and challenges, steadily accompanied by a subtle sense of humor. His service to the University of Virginia included terms as chair of the Department of Chemistry and as Associate Dean of the College of Arts & Sciences. After his May 2016 retirement and appointment as professor emeritus, he continued to teach, and published The Chemical Century: Molecular Manipulation and Its Impact on the 20th Century in 2017. Dick married Lorna Lou Swift, of Madison, South Dakota, on August 19, 1961, and deeply mourned her death in 2001. Together they raised two daughters in Charlottesville while not forgetting where they were from: Most summers saw treks to the Midwest to visit Swift and Sundberg relatives and the family farm in Iowa. Dick's professional success and curiosity also created some special opportunities for the family. His year at Stanford University as an NIH postdoctoral fellow brought family road trips from Virginia to California and back, and visits to dozens of Western state and national parks. A second sabbatical as a Fulbright Scholar moved the family to the suburbs of Paris for a year, and occasioned extensive travel in Europe, including an epic journey to Denmark accompanied by Dick's Iowa-born, Danish-speaking mother, who took her eldest son's family to the home place of her parents, and introduced the Americans to distant but very welcoming Danish cousins.
Dick believed in working hard, and held onto his Midwestern farm boy values of "waste not, want not." He cultivated a Charlottesville community garden plot for decades, raising his plants from seed and harvesting a bounty of food that he cooked and shared. He was a proud and devoted grandfather, traveling to New Hampshire, North Carolina, and beyond for games, performances, and graduations. He was a faithful worshipper, volunteer, and leader at St. Mark Lutheran Church; a loyal friend; a good neighbor; a person of principles; and the author of many firmly worded letters to elected officials. In later years, he enjoyed the loving company of his friend and companion Erin Craner, in gardening, cooking, traveling, and socializing through The Center and the Colonnade Club. He was brilliant, kind, down to earth, patient, organized, generous, hopeful, and utterly practical. To the last, he loved fresh vegetables and extra dessert.
He was preceded in death by his parents, Ernest and Rosa (Christensen) Sundberg; infant brother, Roland Sundberg; sister, Sandra Sundberg Bergman; and wife Lorna Swift Sundberg. He is survived by brother, Ron of Linn Grove, Iowa; brother-in-law, Bud Bergman of Waverly, Iowa; brother and sister-in-law, Dale and Pam Sundberg of San Jose, California; daughters and sons-in-law, the Rev. Kelly Sundberg Seaman and David Seaman of Syracuse, N.Y., and Jennifer and Eddie Deal of Hickory, N.C.; grandchildren, Marshall Deal and wife, Alex, Eleanor Seaman, Emily Deal, Juls Sundberg, Ben Seaman, and Sam Deal, as well as nieces, nephews, and extended family in Iowa, California, Florida, and beyond. The family offers great gratitude to the caregivers and staff members of Carolina Caring, for their hospice care, and of Trinity Ridge, Hickory (Lutheran Services Carolinas), his home from August 2021 until his death.
Due to Covid restrictions, a memorial service will be live-streamed from St. Mark Lutheran Church, Charlottesville, on Monday, December 13, 2021, at 3 p.m. Details will be available at https://stmarklutheran.org. A committal service will be held in Iowa in the spring. Memorial gifts and tributes in lieu of flowers, are gratefully welcomed to the Carolina Caring Foundation, 3975 Robinson Rd., Newton, NC 28658, www.carolinacaring.org/give, or St. Mark Lutheran Church, 100 Alderman Rd., Charlottesville VA 22903. Bass-Smith Funeral Home in Hickory is serving the Sundberg family.
An obituary was also published in UVAToday on Friday, December 3, 2021 and can be found here.
Carl Trindle
Education
B.A. Grinnell College, 1963
Ph.D. Tufts University, 1967
Research in Computational Modeling and Theoretical Chemistry
In the past twenty-five years molecular mechanics and quantum mechanical methods have become essential helpers to experimental chemists of all kinds. Modern software systems teamed with powerful computers now make possible practical representation of many aspects of chemical structure and reactivity. I help newcomers to modeling to develop good judgment in their use of these techniques, and apply some of the most powerful techniques to chemical problems.
I use computer modeling to study reaction pathways in organic systems, structures and energetics of systems likely to possess low-lying states of high spin, and the bonding and reactivity of metal-organic complexes. Much of my work is done in collaboration with graduate students conducting experimental investigations with other UVA faculty.
Some projects I am pursuing now:
- Modeling of aryl carbenes, so to characterize Tomioka’s long-lived methylene species (Presented at the International Symposium on Reactive Intermediates and Unusual Molecules [ISRIUM], Nara Japan, Sept 2001.)
- Characterization of Dearomatization induced by complexation with Osmium, Rhenium, and Tungsten species (presented at the Faraday Discussion York University, April 2003)
- Stereochemical consequences of weak interactions loosely termed “hydrogen bonding” (presented in preliminary form at Rutgers University, February, 2004)
- Characterization of Closed shell and open shell dianions stable with respect to autoionization and dissociation (Presented at ISRIUM, Edinburgh UK August 2005)
- TDDFT characterization of circular dichroism spectra of high symmetry organic species (presented at the ACS National Meeting in New Orleans, April 2008
- Structural characterization of organic cations which bind protons and the resulting dications (to be presented at the Int Conf of Quantum Chemistry, Helsinki June 2009
- TDDFT characterization of fluorescence spectra of Iridium and Ruthenium systems

Computed anharmonic frequencies for Formic Acid Dimers can guide experimental detection of short-lived isomers [figure by Ilhan Yavuz].
Recent Publications
Environmental Sensitivity of Ru (II) Complexes: The Role of the Accessory Ligands, Eileen N. Dixon †, Michael Z. Snow †, Jennifer L. Bon †, Alison M. Whitehurst †, Benjamin A. DeGraff *†, Carl Trindle ‡, and James N. Demas ‡, Inorg. Chem., 2012, 51 (6), pp 3355–3365
Communication: Frequency shifts of an intramolecular hydrogen bond as a measure of intermolecular hydrogen bond strengths, Q Gu, C Trindle, JL Knee, J. Chem. Phys. 2012 137, 091101
Structure and energetics of cyclopropane carboxaldehyde. Trindle, C., Bleda, E. A. and Altun, Z. (2012), . Int. J. Quantum Chem.. doi: 10.1002/qua.24201
Computational thermochemistry of glycolaldehyde. Bleda, E. A., Yavuz, I., Altun, Z. and Trindle, C. (2012), . Int. J. Quantum Chem.. doi: 10.1002/qua.24200
Photophysical and Analyte Sensing Properties of Cyclometalated Ir (III) Complexes, Leavens BB, Trindle CO, Sabat M, Altun Z, Demas JN, DeGraff BA., J Fluoresc. 2012 , 22:163-74
Chuck Arrington
Jan Dean-Clemmer
Jeff Ellena, Ph.D.

Jingyi Li, Ph.D.
Carol Price, Ph.D.

Mark Ross, Ph.D.

Jeffrey Shabanowitz, Ph.D.
I work with Professor Hunt’s group to develop new methods and instrumentation in mass spectrometry to determine the primary structure of proteins and peptides and apply these methods to the structural characterization of proteins in complex mixtures and to peptides presented to the immune system in association with class I or class II molecules of the major histocompatibility complex.

Mallikarjunarao Ganesana, Ph.D.

Gwendoline Stephan, Ph.D.

Zhiyong “Jerry” Zhang, Ph.D.

Wei Chen
Education
B.S. Southwest University of Science and Technology
Ph.D. Sichuan University
Development and application of BINOL-based chiral catalysts and enantioselective fluorescent sensors.

Jerome Ferrance
Education
B.S.E University of Pittsburgh
M.S. University of Pittsburgh
Ph.D. University of Pittsburgh

William Mattson
Education
B.S., University of Virginia
M.S., American University
Ph.D., University of Virginia
Professor Mattson is a member of the faculty at Randolph College in Richmond, VA. He spends his summers here at UVA teaching Introductory College Chemistry.
My passion is teaching. I am one of the richest people on the planet in that I get paid for doing something I thoroughly enjoy.
In all of my classes, I expect my students to work hard and to strive for excellence.
Accomplishments are not measured in how many details are memorized or in how many processes are mastered. What is important is that a true, quality understanding is achieved, such that the student both sees the world with different eyes and can deal with the challenges in her or his future.
To improve my ability to communicate an understanding of chemistry, I have spent the previous fifteen summers teaching general chemistry at the University of Virginia. A memorable piece of student feedback: “I took the chemistry test. I did not know the answers to all of the questions, but what I did not know I could figure out. I made a perfect 800.”
I was invited to present an ACS Webinar on Creative Problem Solving in Chemical Research(May 5, 2011). The ACS weekly webinar series is designed to connect ACS members and scientific professionals with subject matter experts and global thought leaders in chemical sciences, management, and business on relevant professional issues. In addition to presenting the webinar, I have participated in numerous weeklong ACS Speakers Tours, been invited multiple times to speak to several local ACS sections, was invited to be the 27th Annual Harold Hammond Garretson Speaker at Lynchburg College, and have been invited back dozens of times to talk about problem solving in research to undergraduate students at the University of Virginia. In each case, the audience response has been positive, reflecting both their improved insights and abilities in problem solving and the entertaining nature of the talk.
In addition to teaching general, analytical, and instrumental chemistry and to working both on and off campus with students on research projects, I offer a popular course in creative and critical problem solving. The students greatly improve their abilities to think and to solve problems. A memorable piece of student feedback came from a student after a summer: “After I was working on my job for two weeks everyone was calling me MacGyver because I was so good at problem solving.”
I have spent 23 of the past 25 summers teaching or conducting research at the University of Virginia, James Madison University, the University of North Carolina, and the University of Tennessee. I served as a senior reviewer charged with helping to audit all Advanced Placement chemistry courses in the nation, a consultant for the South Carolina Course Alignment Project, a question writer for the American Dental Exam, an Educational Testing Service chemistry Advanced Placement reader, a National Science Foundation panel member for grant evaluation, a director of the Central Virginia Regional Science Fair, and a master of ceremonies for a high school Academic Competition for Excellence program.
It is very important to me that my students like me, but what is most important is that, 10 years into their futures, they are grateful for what they have learned.

Karin Öberg
Education
B.Sc. California Institute of Technology, 2005
Ph.D. Leiden University, 2009
Hubble Postdoctoral Fellow, Harvard-Smithsonian Center for Astrophysics, 2009 – 2012
Organic molecules are found in a diverse set of astronomical environments, demonstrating that there are efficient astrochemical pathways to molecular complexity. Interstellar grains and their icy mantles are proposed to be important formation sites of many of these molecules. Understanding the structure, dynamics and chemistry of ices is thus key to advance our understanding of the chemistry in space.
In the Öberg Astrochemistry Lab we pursue laboratory ice experiments and astronomical observations that address how astrochemically important molecules form, and how they may evolve into larger molecules associated with the origins of life. An important aspect of this research is to constrain the fundamental physical chemical processes that underpin ice chemistry. In addition to exploring the chemical evolution in space, our research forms a basis for developing molecular probes of different astrophysical phenomena.
Recent Publications
Öberg, K.I., Qi, C., Wilner, D. and Hogerheijde, M., Evidence for Multiple Pathways to Deuterium Enhancements in a Protoplanetary Disk, ApJ, 2012, 749, 162
Öberg, K.I., Murray-Clay, R., Bergin, E.A., The effects of snowlines on C/O in planetary atmospheres, ApJL, 2011, 743, L16
Öberg, K.I., Boogert, A.C.A., Pontoppidan, K.M., van den Broek, S., van Dishoeck, E.F., Bottinelli, S., Blake, G.A., and Evans II, N.J., The Spitzer Ice Legacy: Ice Evolution from Cores to Protostar, ApJ, 2011, 740,109
Öberg, K.I., van der Marel, N., Kristensen, L.E., and van Dishoeck, E.F., Complex Molecules toward Low-Mass Protostars: the Serpens Core, ApJ, 2011, 740, 14
Öberg, K.I., Qi, C., Fogel, J.F.J, Bergin, E.A., Andrews, S.M., Espaillat, C., Wilner, D.J., Pascucci, I., Kastner, J., Disk Imaging Survey of Chemistry with SMA (DISCS): II. Southern Sky Protoplanetary Disk Data, ApJ, 2011, 734, 98

Hui Wu
Education
B.S. Southwest Normal University
Ph.D. Lanzhou University

Robert Ellsworth Ireland
Robert Ellsworth Ireland (Bob) passed away on Saturday, February 4th, 2012 at Sarasota Memorial Hospital in Sarasota Florida. He is survived by his wife Margaret, brother Andrew, and children Mark and Richard. Bob, a world-renowned chemistry professor resided in Sarasota these past seventeen years after retiring from the University of Virginia in 1995.
Bob received an A.B. degree from Amherst College in 1951, followed by a Ph.D. degree under the direction of William S. Johnson from the University of Wisconsin in 1954. He then studied as a NSF postdoctoral fellow in the group of William G. Young at the University of California, Los Angeles, from 1954-56. He joined the University of Michigan faculty in 1956 and, subsequently moved to the California Institute of Technology in 1965 as Professor of Organic Chemistry. In 1985, he left Cal Tech to become Director of the Merrell-Dow Research Institute in Strasbourg, France. After a year in that position he came to the University of Virginia as Chairman of the Chemistry department and was selected as the inaugural Thomas Jefferson Chair Professor of Chemistry. He assumed emeritus status in 1995. Among his noteworthy achievements as Chair of the Department was a substantial new addition to the Chemistry Building, completed in 1995.
Bob Ireland received numerous awards in recognition of his contributions to organic synthesis. These include a Sloan Fellowship, the Ernest Guenther Award in the Chemistry of Essential Oils, and the ACS Award for Creative Work in Synthetic Organic Chemistry. He was the first to demonstrate the awesome synthetic potential of the enolate Claisen rearrangement, a reaction that now bears his name. His significant impact on synthetic organic chemistry reflected not only his scientific achievements, but also his style of presentation both in seminars and the scientific literature. His publications are a model of clarity and thoroughness. Early in his career he wrote Organic Synthesis (Prentice Hall, 1969), the first-ever textbook on synthetic strategy. In it one finds the oft quoted passage “Stereochemistry Raises its Ugly Head” as the title of Chapter 5. The final chapter, “Multistage Synthesis: Logistics and Stereochemistry Combine to Produce Nightmares,” presages the present era of complex molecule construction. Though nearly 40 years old, the book can still serve as a text for a modern mid-level course in synthetic organic chemistry.
Bob’s lucid and often entertaining lectures in the classroom and at industrial organizations graphically illustrated the power and beauty of multistage organic synthesis and inspired generations of chemists, both young and old. It is worth noting that many of his former students and postdoctoral associates have become successful chemists themselves and now hold leadership positions in industry, government, and universities, both in the U.S. and abroad. His many contributions to education and science will be long remembered.
James A. Marshall, Charlottesville Virginia

John T. Yates, Jr.
In Memorium
John Thomas Yates, Jr.
August 3, 1935 – September 26, 2015
John T. Yates, Jr., Professor of Chemistry at the University of Virginia, member of the US National Academy of Sciences, and a pioneer of modern surface science, passed away at his home on Saturday morning September 26, 2015, from a recurring glioblastoma. John was both courageous, pragmatic and forthright about his diagnosis right to the end. His wife, Kerin, related that John, upon learning of his diagnosis, said that he had had a great life and was not going to ‘let the last 1.25%’ define him. It did not. He had a wonderful family and a stellar career.
Born in Winchester, Virginia on August 3, 1935, he received his B.S. degree in chemistry from Juniata College in Huntingdon, Pennsylvania in 1956, and his Ph.D. in physical chemistry from the Massachusetts Institute of Technology in 1960 — working with Professor Carl W. Garland. Following three years as an Assistant Professor at Antioch College in Yellow Springs, Ohio, he joined the National Bureau of Standards, Gaithersburg, Maryland (now the National Institute of Standards and Technology), first as an NRC Postdoctoral Research Fellow and then, from 1965 until 1982, as a member of its scientific staff. He was a Senior Visiting Scholar at the University of East Anglia, Norwich, UK in 1970-71, and the Sherman Fairchild Distinguished Scholar at the California Institute of Technology in 1977-78. He joined the University of Pittsburgh in 1982 as the first R.K. Mellon Professor of Chemistry, and as the Founding Director of the University of Pittsburgh Surface Science Center. In 1994 he received a joint appointment in the Department of Physics. In 2006, he retired from the University of Pittsburgh and moved to the University of Virginia as Professor and Shannon Research Fellow.
Throughout his career, his research was in the fields of surface chemistry and physics, including interests in the structure and spectroscopy of surface species, the dynamics of surface processes, and the development of new methods for research in surface chemistry. When he moved to the University of Virginia, he also became professionally active in the field of astrochemistry. He was an accomplished amateur astronomer and woodworker. He had a passionate lifelong interest in clocks, accuracy in timekeeping, and precision instrumentation, both antique and modern. To his core, he was “a measurer” with accuracy and precision. His colleagues and competitors alike knew that they could always trust, without question, the measured quantities in his published works.
John Yates was an exceptional scientist and gifted communicator. He had a knack for making complex problems seem simple after he studied them in depth and communicated his results so beautifully, typically with his own meticulously hand-drawn diagrams. He published more than 750 scientific papers on surface chemistry and physics, and is among the 100 most-cited chemists in the world. His professional accomplishments have been recognized by many prestigious awards and honors, including: Silver Medal – U.S. Department of Commerce (1973); Sherman Fairchild Distinguished Scholar – Caltech (1977-78); Stratton Award for Distinguished Research – NBS (1978); Gold Medal, U.S. Department of Commerce’s Highest Award (1981); Kendall Award for Colloid or Surface Chemistry of the American Chemical Society (1987); Inaugural President’s Distinguished Research Award – University of Pittsburgh (1989); E.W. Morley Medal of the Cleveland ACS (1990); Fellow of the American Physical Society (1992); Medard Welch Award –American Vacuum Society’s highest technical award (1994); Fellow of the American Vacuum Society (1994); Alexander von Humboldt Senior Research Award (1994); Member of the National Academy of Sciences (1996); Pittsburgh-Cleveland Catalysis Society Award (1998); Pittsburgh Award of the Pittsburgh ACS (1998); Arthur W. Adamson Award for Distinguished Service in the Advancement of Surface Chemistry of the ACS (1999); J.W. Linnett Visiting Professorship – Cambridge University (2000); Outstanding Alumnus of Juniata College (2000); G.N. Lewis Lecturer, University of California-Berkeley (2002); Japan Society for the Promotion of Science Fellowship (2002); Gwathmey Visiting Professor, University of Virginia (2002-03); Fellow of The Institute of Physics (2004); and the Peter Debye Award in Physical Chemistry of the ACS (2007); Theodore Madey Award of the AVS (2011); Gerhard Ertl Lecturer Award for Surface Chemistry and Catalysis (2013).
Professor Yates was a kind, patient, trusted and generous mentor and advisor to the more than 1000 students, postdocs and collaborators with whom he interacted. He was an inspirational undergraduate and graduate teacher and mentor. He demonstrated and conveyed an excitement about science, the wonders of scientific discovery, and a love of learning that encouraged and helped many to pursue scientific careers. In addition, he developed strong professional relationships with a number of surface science research programs in academic, government, and industrial research laboratories throughout the world. He served on the editorial boards of six scientific journals and two book series in surface science and catalysis. He was Associate Editor of the ACS journal, Langmuir. He also served on the Advisory Board of Chemical & Engineering News and on the International Advisory Board of Chemistry World.
Yates was generous in his service of scientific societies such as the American Vacuum Society, the American Physical Society, and the American Chemical Society, including past service as a member of the AVS Board of Directors, AVS Trustee Chair, and twice as the AVS Surface Science Division Chair. He was the past chairman of the APS Division of Chemical Physics, and the ACS Division of Colloid and Surface Chemistry. He organized many symposia for ACS and APS national meetings, and was Chairman of three Gordon Research Conferences. He co-edited two books, Vibrational Spectroscopy of Molecules on Surfaces, Plenum, 1987 and Chemical Perspectives of Microelectronic Materials, Materials Research Society, 1989. He co-authored a book entitled, The Surface Scientists Guide to Organometallic Chemistry, ACS, 1987. He also co-authored a textbook, Molecular Physical Chemistry for Engineers, University Science Books, published in 2007. His book, Experimental Innovations in Surface Science, was originally published by Springer-Verlag and The American Institute of Physics in 1998; a second edition, his last major project, was published in 2015.
John is survived by Kerin – his wife of 57 years, his sons Geoffrey (Michelle), and Nathan (Jan), and six grandchildren, Andrew, Steven, Caitlin, Lauren, Hannah and Sara. His passing is a loss for his family and for science, but he has left a lasting legacy in his published work, and in the generations of scientists he mentored.
John N. Russell, Jr., Ph.D.
Head, Surface Chemistry Branch, Naval Research Laboratory, Washington DC
Thomas P. Beebe, Jr., Ph.D.
Professor of Chemistry, University of Delaware, Newark DE
(former graduate students)

William H. Myers
Education
B.A., Houston Baptist University
Ph.D., University of Florida
Bill Myers was a strict teacher teaching a tough subject — chemistry. He expected discipline. However, he would do everything to help his students succeed. He offered review and practice sessions for his exams. He posted every test he ever administered online so students could practice on old tests right before upcoming tests. He recorded his lectures and told stories in class – often funny ones — to help students relate to and understand his subject matter. In a University of Richmond post, Bruce Matthews, assistant athletic director for academics, noted, “He wasn’t going to let you go until you had it. … He just cared.”
Dr. Myers, who retired last May as professor of chemistry at UR after teaching and researching for 43 years, died Sept. 14 at home in Richmond at age 70. Doctors diagnosed an aggressive brain tumor in August. Family members say that chemistry was in his blood from the beginning. William Howard Myers was born Jan. 26, 1946, in a hospital in the shadow of nuclear reactors at Oak Ridge, Tenn., the secret “Fenced City” that housed the Manhattan Project, which the previous year had produced the atomic bomb that won World War II for the Allies.
His chemist father, Albert Myers, who had overseen the chemical aspects of the site’s uranium enrichment program, left to teach at various universities, including Carson-Newman in Jefferson County, Tenn., where Dr. Myers grew up and graduated with honors from high school in 1963. That summer, his father moved the family to Houston, where he helped establish a new Houston Baptist University. Dr. Myers lived at home and attended HBU, majoring in chemistry, physics and math and taking every science course the school offered. After running out of classes to take, he spent his final semester as an intern at Argonne National Laboratory in Chicago, where he decided he never wanted to live again in a cold city.
In Texas, he met Barbara Sue McElvany, whom he married after graduation from HBU in 1967. They moved to the University of Florida at Gainesville, where she finished earning a sociology degree and he began work on his doctorate in inorganic chemistry. When he drew a low draft number during the Vietnam War, Florida put him on staff to teach freshman chemistry, which deferred him long enough to join the Reserve Officer Training Corps and transition into the officers’ Chemical Corps. In 1972, when he earned his doctorate, he was an Army captain. He served in the Army Reserve until 1981.
In fall 1973, he came to the University of Richmond as an associate professor of chemistry, becoming one of five members of his family to teach college chemistry. Dr. Myers chose teaching over research because he loved people and relished encouraging them to reach their potential, according to family. He had a special spot in his heart for student-athletes in his class.
Leland Melvin, a UR football standout who became a NASA astronaut, noted that, “The whole time I was in space, I reflected on people who had not given up on me. … And he was one of them. Dr. Myers was that person who molded me and guided me and helped me understand what chemistry was all about and brought it to life. “All those traits you want your kids to have, he tried to instill them in us: having good character, being a strong person, believing in yourself. It wasn’t just chemistry but in life.”
When former UR offensive lineman Chris Kondorossy became anxious about passing his dental school entrance exam, Dr. Myers invited him to his home, according to the UR post. “I went out there every Saturday for a whole summer at 7 a.m.,” he said. “He privately tutored me for two or three hours. His wife would make us coffee, and we’d sit at the dining room table. “He would go through the textbook front to back. I’d take practice exams with him. I’d get stuck, and he’d tell me why. That’s probably the only reason I was able to get into VCU [Dental School]. … I’ll never forget that. He was looking out for me. That’s who he was.”
Growing up in a family that loved everything from hymns to symphonic music, Dr. Myers developed a love of music and the arts. Playing the lead in “Amahl and the Night Visitors” as a child in Tennessee started him on his way. In Richmond, he sang in the Richmond Symphony Chorus under James Erb and also had lent his voice to the bass sections of church choirs.
In a letter to Dr. Myers on Sept. 6, Jim Hall, a former UR colleague of Dr. Myers, wrote, “I am so glad that you and Barbara took the time this summer to see your families. There’s nothing more important than family this side of Jordan.”
The family archivist, Dr. Myers converted his grandfather’s weekly family newsletters to print and digital formats, preserving more than 25 years of family history. He also collected, organized and preserved family photos and slides.
With Southern Baptist roots running deep on both sides of his family, “he loved his church,” wrote his brother, James “Jim” Myers of Knoxville, Tennessee. “Bill’s life was spent in service to people.”
A self-taught Bible scholar, Dr. Myers found great joy in leading others to more deeply experience God’s word, family members wrote. He taught everywhere he could – at churches, in Kenya, as well as at seminars and conferences. One Thursday per month since 2012, he had led a Bible study group at Deep Meadow Correctional Center. “He was a remarkable teacher of the Bible, of patience, of compassion, of inclusiveness, of love for all people, of ethical/forthright behavior, of clear thinking, of the importance of organization and scholarship, and of unbridled love for God and his family,” another brother, John Myers of Durham, North Carolina, wrote. “His main concern was about each person’s faith in God and their journey with Christ. His gift to each of them was helping them on that journey.”
Please click here to read more on Bill’s life and legacy.
In addition to his brothers, survivors include his wife of 49 years, Barbara Sue McElvany Myers; a daughter, Kathy Burnette; and a son, Bryan Myers, both of Midlothian; and one grandson.
September 20, 2016, Ellen Robertson, Richmond Times Dispatch

Victoria Holt
Victoria is from Herndon, Virginia and is studying chemistry with a minor in Russian language and literature. She began research with Dr. Landers in the fall of 2015, working with Shannon Krauss on an explosives detection project. The project is working to develop a portable device in which colorimetric reactions occur in the presence of various explosive materials. Reactions for nitrates, hydrogen peroxide, perchlorates, TNT, DNT, and tetryl have been implemented on the device. Victoria focused most of her work on the nitrates. By modifying the Griess reaction, which turns bright pink when reagents form an azo dye with nitrate, the reaction and limits of detection were optimized for use in the field.
When she isn’t working in the lab, Victoria loves to dance, read, and cook. She has been a member of University Dance Club throughout her college career, and joined University Salsa Club during her third year. She also volunteered as an elementary school tutor through Madison House and was a fundraiser for Dance Marathon for UVA Children’s Hospital.

Nikki Aaron
Nikki grew up in Fairfax, Virginia and attended Robinson Secondary School. She will be graduating from UVA with a Bachelor of Arts in Chemistry and a Minor in Psychology. Since August 2015, Nikki has been working in Dr. Thurl Harris’ lab in the Pharmacology Department in the School of Medicine. Dr. Harris’ lab studies how the enzymes involved in lipid metabolic pathways are regulated and how their deregulation can lead to pathological conditions such as obesity and Type II Diabetes. Over the last two years, Nikki has been exploring the stability of lipin-1, a phosphatidic acid phosphatase enzyme in the Kennedy pathway of lipid synthesis and an essential component of triacylglycerol synthesis in adipose tissue. She is working to identify critical residues that regulate lipin-1 ubiquitination and degradation by the proteasome. In light of the obesity epidemic, understanding what regulates lipin-1 stability and its ability to synthesize triacylglycerol may help to illustrate the mechanisms at play in metabolic derangements that physicians see every day. Following graduation, Nikki will be pursuing a Ph.D. in Pharmacology at Columbia University in New York City.

Jack Cronk
Jack Cronk grew up in Charlottesville, Virginia and graduated from Western Albemarle High School. He is currently pursuing a Bachelors of Science in Chemistry with a specialization in Biochemistry along with ACS certification. Jack conducts research in the School of Medicine in the laboratory of Dr. Michael Brown, Ph.D. Over the last 3 semesters, Jack has designed and utilized genetic approaches to systematically and specifically induce mutations in the genes of natural killer (NK) cell receptors. NK cells play an essential role in the innate immune response as cytotoxic lymphocytes that recognize cells infected with virus. Jack’s research investigates genetic mutations in NK cell receptors, the effects of which will ultimately be evaluated with respect to how these mutations impact receptor functionality. His thesis focuses on the validation of a meticulous experimental approach to study the immediate impact of these gene disruptions. Current and future work will involve testing his experimental design and evaluating the effects of the receptor mutations at the genomic level. Following graduation, Jack plans to continue his research at UVa, spend time with his two miniature dachshunds, Piper and Elly, and eventually pursue a Ph.D in Immunology.

Maggie Daly
Maggie grew up in Yorktown, VA, but went to high school at the International School of Provence-Alpes-Cote-D’azur in Manosque, France. She is pursuing a Bachelor of Science with Specialization in Biochemistry and a Minor in Religious Studies with a concentration in Islam.
Since May 2015, Maggie has been conducting research with Dr. Cassandra Fraser in the Department of Chemistry. The Fraser Lab studies the synthesis and applications of dual-emissive polymeric materials for oxygen sensing and imaging in biomedical contexts such as tumors, wounds and the brain. The dyes are luminescent difluoroboron b-diketonates that are covalently linked or blended with a biocompatible polymer, such as poly(lactic acid), and precipitated into nanoparticles. Maggie’s projects in the Fraser Lab have a focus on the molecular design of the luminescent boron dyes, with efforts to elucidate the trends for tuning their optical properties.
Outside of the lab, Maggie is an active member of Club Swim as well as Alternative Spring Break at UVA. She has also worked as an Organic Chemistry Lab TA for the past two years. Next year, she will begin pursuing a PhD in Materials Science at the University of North Carolina at Chapel Hill.

Anna Perkins
I am from Atlanta, GA, and I went to The Lovett School. I will graduate with a BSc in Chemistry and a BA in Studio Art, painting concentration. I stated research with the Demas lab Spring 2016, focusing on fluorescence anisotropy. I have worked with the oxygen sensor Ru(bpy)3, Fraser’s promising boron complex nanoparticles, and fluorescent dye-polymer equilibria that model biological binding systems. Measuring the anisotropy of these compounds gives valuable information about the excited state(s) and information about binding. Fluorescence anisotropy is the study of emission polarization and is commonly used to measure the binding of biological equilibria. When a polarized excitation source excites a fluorophore, that fluorophore can emit in the same orientation, or it can rotationally diffuse before it has the chance to emit, thus producing an unpolarized emission. Anisotropy is a ratiometric measurement of the extent of the emitted polarization.
In addition to painting, I sing in two different groups on Grounds, the Harmonious Hoos co-ed a cappella group and the Virginia Women’s Chorus.

Youlim Ha
Youlim was born in Seoul, South Korea and grew up in Nanjing, China. She is graduating with the Bachelor of Science in Chemistry with the ACS certification from the University.
Youlim is doing research with Professor Ku-Lung (Ken) Hsu in the Department of Chemistry. In order to understand protein function in human disease and physiology, the lab develops novel small molecules that enable molecular analysis of proteins with mechanistically-related reactivity and activity. Her research is focused on the synthesis of new chemical probes to identify novel small molecule binding pockets present in proteins that were previously undetected due to a lack of suitable chemical biology approaches.
She received the Undergraduate Poster Session Award from the American Chemical Society for her research. She also received the Department of Chemistry Award for Excellence in Chemistry. In the fall, she is moving to London for studying Advanced Chemical Engineering.

Ellen Howerton
Ellen Howerton grew up in Fairfax, Virginia and graduated from Thomas Jefferson High School for Science and Technology. She will graduate from UVa with a Bachelor of Science in Chemistry with a biochemistry specialization.
Ellen joined the Bushweller lab group in the Molecular Physiology and Biological Physics department in January, 2015. For her distinguished major, she focused on the Ets-domain family of transcription factors, which are deregulated in multiple cancers. Many Ets-domain proteins exhibit autoinhibition, a phenomenon that occurs when a separate portion of a protein inhibits the function of another domain. As the autoinhibitory domain is likely unique between Ets family members, it provides a promising target for therapeutics.
Outside of her studies, Ellen is a violinist and an active member of Radio Music Society, a student-run group that writes and performs string quartet covers of popular songs. She is also a Soprano 1 with the Virginia Women’s Chorus and a member of the Washington Literary Society and Debating Union. After graduation, Ellen will be working at the National Human Genome Research Institute at the NIH and later hopes to pursue graduate study in Public Health.

Daniel Mulrow
Daniel grew up in Arlington, Virginia and attended Washington-Lee High School. He will be graduating UVA with a Bachelor of Science in Chemistry with a Specialization in Chemical Physics and a Bachelor of Arts in Physics.
Since August of 2013 Daniel has been working for the Demas research group. His primary focus has been using fluorescence anisotropy to determine binding constants between fluorescent dyes and polyelectrolytes. This method has been shown to allow for more types of binding constants to be measured than using other fluorescent techniques. It also has shown that more complex binding occurs when the polymer concentration is much greater than that of the fluorescent dye.
Outside of lab, Daniel is very involved with UVA’s honor fraternity Phi Sigma Pi as well as being a First Year Seminar Facilitator for the Orientation and New Student Programs office at UVA. He has also served as a physical chemistry teaching assistant for the past year. After graduation, Daniel will be pursuing a Ph.D in nuclear/physical chemistry.

Joshua Corbin
Joshua attended Franklin County High School in Rocky Mount, VA. He is pursuing a B.Sc. in Chemistry with a Specialization in Biochemistry with ACS certification.
His research is in the lab of Dr. Lin Pu where he worked on a project with graduate student Shifeng Nian synthesizing a bimetallic catalyst to control the tacticity in atomic transfer radical polymerizations (ATRP) of functional alpha-olefins (e.g. acrylamide) by synthesizing a salen-derived macrocyclic ligand to coordinate a Lewis acidic metal and copper. Previous research showed that addition of a Lewis acid to Cu-mediated ATRP was promising for producing steroregular polymers. We confined the Lewis acidic center and the copper center in close proximity on a macrocyclic ligand to couple their roles in the polymerization and enhance the catalytic efficiency. Our work has provided evidence that these bimetallic catalyst systems incorporate a cooperative effect utilizing the Lewis acidic monomer activation with the copper-chlorine radical generation and stabilization process in order to provide stereocontrol and catalysis to the ATRP processes. Following the introduction of chirality into the catalyst system, the reaction of the Lewis acid coordinated monomer with the adjacent transient free radical, generated from the copper-chlorine abstraction at the polymer end, proceeds with significant stereocontrol to give the desired isotactic polymers. We are still working to optimize the degree of monomer conversion and stereocontrol.
In addition to research, Joshua has been a teaching assistant for CHEM 3410 and CHEM 3420 (physical chemistry, thermodynamics and quantum chemistry) under Dr. Dave Metcalf. Though still undecided on his particular focus, he will study organic, organometallics, or polymer chemistry in graduate school.

Andrew Lankenau
Andrew was born in Silver Spring, MD but attended Oakton High School in Vienna, VA. He will be graduating from UVA with a Bachelors of Science in Chemistry. Since January 2012, Andrew has worked in the research group of Dr. W. Dean Harman.
For the majority of his time in the Harman group, Andrew’s project has been to separate the two enantiomers of a chiral tungsten dearomatization core. To do so, a tartaric acid derivative was first used to create two diastereomeric salts. From there, the salts were separated via a finely tuned precipitation reaction and then returned to their original state by removing the asymmetric anion with a base. This research presents a novel approach for the enantio enrichment of chiral organometallic complexes. Andrew is in the process of submitting a first author publication of this research and hopes to have it published before he graduates.
Outside of academics, Andrew is a dedicated fan of UVA basketball and religiously attends all home games. After graduation, Andrew will be pursuing a Ph.D in inorganic chemistry.

Nick Lee
Nick Lee is a fourth-year Distinguished Major in Biochemistry from Winchester, Virginia. He conducts research in the School of Medicine in the laboratory of Anindya Dutta, M.D., Ph.D., where he investigates the function of the CRL4(Cdt2), an E3 ubiquitin ligase, in the cell cycle. The complex is responsible for marking cell cycle regulators for degradation by the proteasome. Previously, he worked to elucidate the stabilizing role that 14-3-3 exerted over Cdt2. His thesis is focused on characterizing the interaction between BRAF35 and Cdt2. BRAF35 is relatively uncharacterized in the literature, but it interacts with BRCA2, the breast cancer susceptibility protein. For his research endeavors, he has received a U.Va. Summer Scholars Award, a Harrison Undergraduate Research Award, a College Council Fall Research Grant, and a Small Research and Travel Grant, along with being a published co-author in Molecular and Cellular Biology.
Currently, he serves as the Vice Chair for Trials for the Honor Committee, the Chair of the Undergraduate Research Network, and the President of the College Science Scholars Council. Outside of those commitments, Nick has taught his own CavEd class, Current Topics in Neuroethics, served as a TA for Organic Chemistry, and is an Echols Scholar, College Science Scholar, a member of the Raven Society, and a Lawn Resident. After graduation, he will be pursuing his M.D. with the desire to become a professor of medicine.

Emily Schutzenhofer
Emily M. Schutzenhofer is from Stafford, Virginia and is a graduate of Colonial Forge High School. She is an Echols Scholar double majoring in Chemistry with a Specialization in Biochemistry (including the ACS certificate) and Global Development Studies with a concentration in Global Public Health.
Emily conducts research in the lab of Dr. Gary K. Owens in the Robert M. Berne Cardiovascular Research Center. She studies the molecular mechanisms controlling the expression of an embryonic stem cell pluripotency gene, Oct4, in adult smooth muscle cells. Of particular interest to her are those mechanisms involving the vessel environmental cues typically associated with the development and progression of atherosclerotic lesions. The implications of her research include increasing understanding of the development of atherosclerosis, a condition characterized by the hardening of arteries due to the buildup of plaque. End-stage, catastrophic clinical events associated with atherosclerosis include myocardial infarction and stroke, provoked by plaque rupture and major thrombotic events. In addition, her research contributes to the field of knowledge surrounding smooth muscle cell phenotypic switching— control of which could constitute even more widely applicable clinical interventions related to cardiovascular diseases.
Outside of her academic pursuits, Emily proudly serves as the President of the National Leadership Council of the National Society of Collegiate Scholars, one of the nation’s largest and most prestigious college honor societies. She also serves on the Board of Directors for the Society. Emily is an aspiring physician and, as such, is passionate about health, wellness, and service— in addition to her research at the CVRC, she volunteers at the Charlottesville Free Clinic and Remote Area Medical Clinics in underserved regions of the state, has led the Women in Medicine Initiatives interest and advocacy group at UVA, has studied and participated in research on public health interventions in developing nations to improve chronic asthma management, and has founded and leads a service organization, the Virginia Cyber Leo Club at UVA, to help people with disabilities in the local community.

Francis Collins
Dr. Collins earned a B.S. in Chemistry at the University of Virginia in 1970 (mentor Carl Trindle). He went on to attain a Ph.D. in physical chemistry at Yale University in 1974. He then enrolled in medical school at the University of North Carolina at Chapel Hill, earning there an M.D. in 1977. From 1978 to 1981, Dr. Collins served a residency and chief residency in internal medicine at North Carolina Memorial Hospital in Chapel Hill. He then returned to Yale, where he was named a Fellow in Human Genetics at the medical school from 1981 to 1984. During that time, he developed innovative methods of crossing large stretches of DNA to identify disease genes. In 1984, Dr. Collins joined the University of Michigan in a position that would eventually lead to a Professorship of Internal Medicine and Human Genetics. Dr. Collins accepted an invitation in 1993 to succeed James Watson as Director of the National Center for Human Genome Research, which became NHGRI in 1997. As Director, he oversaw the International Human Genome Sequencing Consortium. Dr. Collins’ accomplishments have been recognized by numerous awards and honors, including election to the Institute of Medicine and the National Academy of Sciences, and the Presidential Medal of Freedom. On July 8, 2009, President Barack Obama announced he will nominate Dr. Collins to lead the National Institutes of Health. On August 17, 20019, he was sworn in as the 16th Director of the NIH and was asked to continue by both Presidents Trump and Biden. The NIH Biographical Sketch of Dr. Collins can be found here.

Craig Crews
Professor Crews earned his Chemistry B.A. at the University of Virginia in 1986. He then studied in Germany at the University Tübingen with a German Academic Exchange Service (DAAD) fellowship. He earned his Ph.D. in Biochemistry at Harvard University in 1993. After a postdoctoral fellowship with Stuart Schreiber at Harvard he joined the Yale Molecular, Cellular, and Developmental Biology Department faculty. He is also a faculty in the Departments of Chemistry and Pharmacology at Yale. His laboratory investigates chemical approaches to the study of biological questions and is specifically interested in the modes of action of biologically active natural products in order to investigate intracellular signaling pathways and identify novel targets for drug design. In 2006, Prof. Crews received the Friedrich Wilhelm Bessel Award from the Alexander von Humboldt Foundation and, in 2009, he received a Grand Challenges Exploration grant from the Bill & Melinda Gates Foundation.

Cynthia S Dowd
Professor Dowd earned her B.A. degree from the University of Virginia and her Ph.D. in Medicinal Chemistry from Virginia Commonwealth University (working with Richard Glennon). Following postdoctoral work at the University of Pennsylvania (with Irwin Chaiken), she joined the NIH where she directed a synthetic chemistry group finding novel small molecules against Mycobacterium tuberculosis. In 2007, she joined the Chemistry Department of George Washington University as an assistant professor. Her research interests broadly include anti-infective drug discovery, structure-based ligand design, and the development of chemical tools to understand important biological processes.

Christopher B. Ferenc
After graduating from UVa with a B.S in Chemistry, Christopher Ferenc received his law degree from Seton Hall University School of Law, in Newark, NJ. His background in chemistry motivated him to pursue a career in the field of intellectual property law. His professional experience in this field includes interning with a U.S. Magistrate Judge and serving as legal support staff for a U.S. Congressional Committee. Currently, he is employed as a Patent Attorney in Washington, D.C. His practice focuses on the preparation and prosecution of U.S. and foreign patent applications in the chemical arts, as well as providing counsel to clients on issues relating to invalidity, infringement and patentability of U.S. patents.

J. Christopher Love
Professor Love is an assistant professor in chemical engineering at MIT. He is also an associate member at the Eli and Edythe L. Broad Institute, and associate faculty at the Ragon Institute of MGH, MIT, and Harvard. He was named a Dana Scholar for Human Immunology and a Keck Distinguished Young Scholar in Medical Research in 2009.
Professor Love graduated with a B.S. degree in chemistry from the University of Virginia in 1999 (conducted research with Cassandra Fraser). He received his Ph.D. in 2004 in physical chemistry at Harvard University under the supervision of George Whitesides. Following completion of his doctoral studies, he extended his research into immunology at Harvard Medical School with Hidde Ploegh from 2004-2005, and at the Immune Disease Institute from 2005-2007. His current research uses microsystems to characterize heterogeneity among single cells with specific studies in HIV/AIDS, autoimmunity, and biopharmaceutical manufacturing. He was selected as on of a Brilliant 10 for 2010 in PopSci.

Brian Pollok
Brian Pollok, Ph.D., is Life Technologies’ Chief Scientific Officer and Head of Global Science & Innovation based in Carlsbad, CA. He oversees the allocation of more than $350M in R&D funds annually, which has yielded innovative new products in the areas of DNA sequencing, cell analysis, and molecular biology. Dr. Pollok has been with Life Tech since 2003, previously serving as CSO and Head of Global R&D for Invitrogen, and as VP of R&D for the company’s Discovery Sciences unit in Madison, WI. Prior to joining Life Tech, Dr. Pollok served as Sr. VP of R&D and Co-Founder at Ansata Therapeutics in La Jolla (2002-03), as VP of Discovery Biology at Aurora Biosciences/Vertex Pharmaceuticals in San Diego (1997-2002), as Sr. Research Investigator at Pfizer Central Research in Groton, CT (1993-97), and as Assistant Professor of Microbiology and Immunology at Wake Forest University in Winston-Salem, N.C. (1987-93). Dr. Pollok received his B.A. in biology and chemistry from UVa, and his Ph.D. from UAB. Dr. Pollok held a Damon Runyon Cancer Fund postdoctoral fellowship at the Fox Chase Cancer Center, Philadelphia. He is a member of the editorial board of the journal ASSAY, and is an advisor for several non-profit disease organizations. Dr. Pollok is also a past recipient of an American Cancer Society Faculty Research Award and an Arthritis Foundation Investigator Award.

Christian M. Rojas
Professor Rojas earned his B.A. from the University of Virginia in 1989, where he did research with Ralph O. Allen on neutron activation analysis of archaeological artifacts. He received a Ph.D. in organic chemistry from Indiana University, working with David R. Williams. Following a postdoc with Julius Rebek at both MIT and The Scripps Research Institute, Professor Rojas joined the faculty at Barnard College, a liberal arts college for women in New York City. His research involves the development of nitrogen atom transfer reactions and their application to the synthesis of 2-amino sugars.

Eric A. Rohlfing
Dr. Rohlfing is the division director of the Chemical Sciences, Geosciences and Biosciences Division in the Office of Basic Energy Sciences (BES), Office of Science, U.S. Department of Energy. He joined BES in 1997 and served as program manager for the Atomic, Molecular and Optical Sciences program from 2000 to 2003 and as team leader for Fundamental Interactions from 2003 until October 2006, when he became division director. Dr. Rohlfing received his B.S. in Chemistry from the University of Virginia in 1977 and his Ph.D. in Physical Chemistry from Princeton University in 1982. He was a postdoctoral fellow at Exxon Research and Engineering Company and Los Alamos National Laboratory before joining the staff at the Combustion Research Facility at Sandia National Laboratories in 1986.
His research interests include the experimental characterization of transient molecules relevant to combustion processes, linear and nonlinear laser spectroscopies, trace detection of pollutants, molecular beam and mass spectrometric studies of carbon and metal clusters, and vibrational relaxation dynamics. He is the author of approximately 50 peer-reviewed articles, holds membership in the American Chemical Society and the American Physical Society, and is a fellow of the American Association for the Advancement of Science.

Stuart L. Schreiber
Director of Chemical Biology at and Founding Member of the Broad Institute of Harvard and MIT, where he is a Investigator. He is also the Morris Loeb Professor of Chemistry and Chemical Biology at Harvard University. He is a member of the National Academy of Sciences and the American Academy of Arts & Sciences (1995).
Dr. Schreiber was born February 6, 1956 and raised in Virginia. After receiving a B.A. degree (conducting research with Richard Sundberg) at the University of Virginia in June of 1977, he carried out graduate studies at Harvard University under the supervision of R. B. Woodward and Y. Kishi. Following completion of his doctoral studies, he joined the faculty at Yale University in May of 1981. He was promoted to Associate Professor with tenure in 1984 and to Full Professor in 1986. In 1988, he returned to Harvard.
Dr. Schreiber is known for having developed systematic ways to explore biology, especially disease biology, using small molecules and for his role in the development of the field of chemical biology. Currently, the Schreiber group research is focused on:
- Development of next-generation synthetic chemistry affording a transformative small-molecule screening collection.
- Investigating small molecules using human primary cells in an environment that mimics their in vivo niche.
- Exploiting the remarkable ability of genetic approaches to illuminate the roles of genes in biology and disease
- Attempting to discover small molecules that increase pancreatic beta cell numbers and function using organ cultures of human primary pancreatic islets

Webster Santos
Professor Santos earned his B.S. degree from the University of Virginia and continued at UVA for his Ph.D. studies with Timothy Macdonald. After graduating in 2002, he was an NIH Postdoctoral Fellow at Harvard University in the Department of Chemistry and Chemical Biology with Professor Gregory L. Verdine. He is now an assistant professor at Virginia Tech.

Robert Sell
After Robert Sell earned his B.S. in Chemisty with High Distinctions from UVA in 1975, he worked for four years in the U.S. Navy as an instructor at the Naval Nuclear Power School teaching Chemistry, Radiological Fundamentals, and Materials Science. From 1979 to 2009, he worked with Corning Incorporated in a wide variety of positions in manufacturing, process engineering, product and market development, strategy development, intellectual property, product line management. During his time at Corning, he obtained a Masters in Business Administration from West Virginia University in 1987. Over the 30 years he worked at Corning , he participated in the areas of glass products for the pharmaceutical industry, environmental laboratory analysis, and semiconductor manufacturing. He is now retired from Corning and instructing at Corning Community College.

Jeff Toney
Dr. Toney’s career has spanned both the pharmaceutical industry and academia. His academic training is in Chemistry (B.S., University of Virginia; M.S. and Ph.D., Northwestern University) and included research experience as a postdoctoral fellow in Molecular Biology (Dana Farber Cancer Institute, Harvard Medical School) and in Chemical Biology (Massachusetts Institute of Technology).
While at UVA, Dr. Toney conducted research with James Demas, which resulted in a publication (Toney, J.H., Demas, J.N. “Low frequency computerized lock-in amplifier”, Rev. Sci. Inst., 53: 1082-1085 (1982)).
His current scholarship is focused on drug discovery using an interdisciplinary approach. As a Senior Research Fellow at Merck Research Laboratories, he studied a variety of therapeutic targets for which high throughput biochemical assays were developed. He has held the Herman and Margaret Sokol Professorship in Chemistry at Montclair State University and served as Department Chairperson of Chemistry and Biochemistry. During this time, he developed a new graduate course, “Biomolecular Assay Development” that emphasizes teaching of the drug development process, including laboratory and in silico molecular modeling techniques. He is a member of the Editorial Board of Assay and Drug Development Technologies and is a Section Editor of Current Opinion in Investigational Drugs. He has served ten times as a member of the review panel, “Assay Development for High Throughput Molecular Screening” (R03, R21) of the National Institutes of Health, Molecular Libraries and Imaging Initiative. He has published 51 peer reviewed articles and holds six U.S. patents. Dr. Toney joined Kean University in 2008 as Dean of the College of Natural, Applied and Health Sciences. Dr. Toney is Vice President for Academic Affairs (2011- present) at Kean University. He is currently serving as the Chief Academic Officer (since 2011) and Provost and Vice President for Academic Affairs at Kean University and is continuing interdisciplinary research in drug discovery and has been appointed as Visiting Professor at MIT in the Department of Linguistic and Philosophy for Summer 2019..

Kian Tan
Professor Kian Tan graduated with a B.S. in chemistry with a specialization in biochemistry from the University of Virginia in 1999. At UVa, Kian performed research in the group of Professor Dean Harman working on the development of an osmium-mediated asymmetric Diels-Alder reactions and the synthesis of epibatidine derivatives as analgesics. Subsequently, Kian worked jointly with Professors Robert Bergman and Jonathan Ellman at the University of California Berkeley on novel metal-mediated C-H activation reactions. He obtained his Ph. D. from UC-Berkeley in 2004. Working as a postdoctoral researcher in Professor Eric Jacobsen’s group at Harvard University, Kian focused on bifunctional urea catalysts for the enantioselective allylation of hydrazones. In 2006, Kian began as an Assistant Professor at Boston College where he enjoys teaching organic chemistry and guiding a research program focused on the new catalysts for the transformation of organic molecules.

Ann M. Valentine
Professor Valentine earned her B.S. in Chemistry from the University of Virginia. She conducted undergraduate research with Timothy Macdonald on aluminum inhibition of magnesium-dependent enzymes. After graduating in 1993, she went to MIT to earn a Ph.D. with Steve Lippard. She then conducted her postdoctoral research at Penn State University and, in 2001, joined the Yale Departmet of Chemistry faculty. Her research explores the use of metals in nature and the development of potential titanium-based anticancer drugs. She has received numerous awards including the American Chemical Society PROGRESS/Dreyfus Lectureship Award in 2007 and the Paul D Saltman Award for Metals in Biology in 2009.

Erskine Williams
Erskine Williams was born and raised in Richmond, Va. He graduated from the University of Virginia in 1996 with degrees in Biochemistry & Cognitive Science. After graduating, he moved to Hood River, OR for two years to windsurf. In 1998, he moved to Portland, OR and started software engineering for Intel. From 2000 -2001, he rode the dot.com bubble with a small consulting firm in San Francisco. When the bubble popped, Erskine worked for Barclays Global Investors as a software engineer in San Francisco from 2001 – 2003. In 2003, he married and moved back to Portland working with Fujitsu Biosciences to write 3D molecular modeling software. Then, in late 2005, Erskine joined a company which brings social networking software to businesses.

Henry A. Boyter, Jr.
Henry Boyter Jr. is the Director of CESTAB (Center for Environmentally Sustainable Textile and Apparel Businesses). His research and industry service is directed at the application of green chemical techniques to textile processes. He is the past Chair of the AATCC RA100 Global Sustainability Technology Research Committee. He is a former member of the Peer Review Group for the American Apparel & Footwear Association (AAFA) Restricted Substances List Task Force and was a Joint Committee (JC) member developing a “Sustainability Assessment for Commercial Furnishings Fabric – NSF/ANSI 336 – 2011” under the Association for Contract Textiles (ACT) and NSF International and was Leader of the Water Group. He is currently involved with an industry task force under the direction of NTA to Update the VPEP form used by industry for chemical information exchange and is working on the Outdoor Industry Association’s (OIA) Eco-Index as a member of the Toxics Subgroup.He is the author of “Environmental Legislation USA” In Environmental Aspects of Textile Dyeing; Woodhead Publishing Limited.

John M. Butler
John M. Butler is NIST Fellow and Group Leader of Applied Genetics at the National Institute of Standards and Technology. He is author of the internationally acclaimed textbook Forensic DNA Typing—now in its third edition—as well as more than 100 scientific articles and invited book chapters. His book was also been translated into Chinese (2007) and Japanese (2009). He earned his Ph.D in 1995 from the University of Virginia with Ralph Allen (Analytical Chemistry). His Ph.D. research was conducted in the FBI Laboratory, involved pioneering the techniques now used worldwide in modern forensic DNA testing. Over the past 15 years, Dr. Butler has worked in government and industry. He designed and maintains STRBase (http://www.cstl.nist.gov/biotech/strbase), an information resource for short tandem repeat DNA markers. As a member of the World Trade Center Kinship and Data Analysis Panel, he aided the New York City Office of Chief Medical Examiner in their work to identify the remains of victims of the 9/11 terrorist attacks. He also serves on the Department of Defense Quality Assurance Oversight Committee for DNA Analysis and advises numerous national and international forensic DNA efforts. Dr. Butler has received numerous awards during his career including the Presidential Early Career Award for Scientists and Engineers (2002), the Department of Commerce Gold Medal (2008) and Silver Medal (2002), the Arthur S. Flemming Award (2007), Brigham Young University’s College of Physical and Mathematical Sciences Honored Alumnus (2005), and the Scientific Prize of the International Society of Forensic Genetics (2003). In August 2011, ScienceWatch.com announced that Dr. Butler was number one in the world as a high-impact author (number of citations per paper published) in legal medicine and forensic science for the decade of 2001-2011.

Christopher D. Claeboe
After earning his Ph.D. with Sidney Hecht at the University of Virginia in 2003, Dr. Christopher Claeboe joined the laboratory of David R. Williams at Indiana University for a post-doctoral fellowship that was focused upon the total synthesis of Peloruside A. In 2005, Dr. Claeboe began his industrial career as a process chemist with Albemarle Corporation, working at their Baton Rouge, LA facility. He then transferred to their South Haven, MI facility in 2008 where he currently resides. While responsible for the completion of a variety of interesting side projects for the company including the pursuit of his MBA through Indiana University, his work is centered upon the process development and scale-up of custom active pharmaceutical ingredients ().

April Frazier
Dr. Frazier graduated from Harvey Mudd College with a B.S. in Applied Chemistry. In 2003, she earned her Ph.D. from the University of Virginia Chemistry Department with Prof. David Cafiso. She works at Pro-Cure Therapeutics (a Prostate Cancer Stem Cell Company) where she performs two roles. She is the Commercial Operations Manager and a research scientist. She manages business relationships with corporate partners and tests and develops assays for novel prostate cancer stem cells.

Benjamin A. Garcia
Professor Garcia received his B.S. degree from the University of California, Davis and then went on to earn his Ph.D. from the University of Virginia under Donald Hunt in 2005. Ben then was an NIH NRSA Postdoctoral Fellow at the Institute for Genomic Biology at the University of Illinois, Urbana-Champaign with Professor Neil Kelleher. Currently, he is an Assistant Professor of Molecular Biology and Chemistry, and a member of the Quantitative and Computational Biology program at Princeton University where he has received a 2010 NIH Director’s New Innovator award.

David McWhorter
Dr. McWhorter earned a Ph.D. in Chemistry (with Brooks H. Pate) from the University of Virginia and a B.S. in Chemistry from Washington and Lee University.
After a postdoctoral appointment at The National Institute for Science and Technology (NIST), Dr. McWhorter spent over seven years at the Institute for Defense Analyses (IDA), a Federally Funded Research and Development Center. There, in addition to several Department of Defense projects, he served as the Deputy Project Leader for IDA on the US Department of Homeland Security’s (DHS) SAFETY Act (the Support Anti-terrorism by Fostering Effective Technologies Act of 2002). In this position he led the technical evaluations of anti-terrorism products and services across a broad spectrum of countermeasures for chemical, biological, explosive, nuclear, cyber and human threats, including services.
In 2007, Dr. McWhorter joined Catalyst Partners, a DC-based homeland security consulting and government relations firm. In his role as a Principal, he began Catalyst’s SAFETY Act Practice and Technology Evaluation Practice. He represented clients in front of DHS, private sector companies, and other potential customers, spearheading dozens of successful SAFETY Act applications.
Dr. McWhorter started his own firm in 2014: The Homeland Security Consulting Group (HSCG). Working with industry partners, The HSCG continues serving clients across the Homeland Security space. He constantly scouts the anti-terrorism and security marketplaces to identify relevant technology, performs independent evaluations, and helps his clients assemble quantitative evidence of the effectiveness of the technologies. The HSCG also provides business development services for its clients. Leveraging his vast network of security professionals, Dr. McWhorter brings his clients’ technology directly to potential end users and to potential industry partners. In the years since The HSCG was founded, Dr. McWhorter has prepared several more dozen successful SAFETY Act applications for his clients. Representative clientele includes Fortune 500 companies, start-up tech companies, and everything in between.


Seth W. Snyder
Seth W. Snyder, Ph.D. received a B.A. from University of Pennsylvania in Chemistry and Environmental Studies (1980), a M.S. in Physical Chemistry (1985) and a Ph.D. in Biophysics (1989) from the University of Virginia in James Demas‘ laboratory. He completed a postdoctoral fellowship at Argonne National Laboratory in Photosynthesis. In 1989, he joined Abbott Laboratories, first in Alzheimer’s Disease Research and later in Pharmaceutical Discovery Research. In 1998, Seth rejoined Argonne as the Associate Director of the Chemistry Division where he developed new programs in nanoscience and applied biotechnology. In 2001, he joined the Energy Systems Division as the Section Leader of Chemical and Biological Technology and now Process Technology Research. His team develops new process technologies ranging from tree growth through conversion technologies and product separations. The goal is to improve energy efficiency in production of biofuels and biobased products, CO2 capture, and water treatment. In other technology areas, his team works on plastic recycling, PV materials, geothermal energy, and now battery materials.
He serves as the President of the Council for Chemical Research and as Argonne’s Lab Relationship Manager for the DOE Office of the Biomass Program. He is on the advisory board for several academic centers including: the University of Illinois Urbana-Champaign’s “Center for Advanced Bioenergy Research”, South Dakota’s “Center for Bioprocessing Research”, and the NSF “Center for Bioenergy R&D”. He has published about fifty papers, has twelve patents (issued and pending), has presented or co-authored his research at 75 conferences over the past nine years. He has received three R&D 100 awards and an Outstanding Mentor Award from the DOE FAST Program.

Steven Shipman
Professor Shipman received his B.A. from Rice University and earned his Ph.D. from the University of California-Berkeley under Charles Harris in 2005. He was then a post-doctoral researcher from 2006 until 2008 at the University of Virginia with Professor Brooks Pate. He is currently an Assistant Professor of Physical Chemistry at New College of Florida, a small liberal arts college in Sarasota, FL. His research is concerned with the study of the rotational spectra of molecules in vibrationally-excited states at room temperature.

Scott C. Bailey-Hartsel
Professor Scott C. Bailey-Hartsel received his B.S. degree from Ohio University and then went on to earn his Ph.D. from the Ohio State University in 1985. He worked as an American Heart Association postdoctoral fellow under David Cafiso from 1985-1987 at UVa and was an NSF/CNRS research fellow at Universite Pierre et Marie Curie and Institut Curie in Paris in 1995 and 96. Scott is currently Professor and immediate past Chair of Chemistry at the University of Wisconsin-Eau Claire, a member of the ACS Committee on Professional Training (CPT) and was a past member of the Council on Undergraduate Research (CUR). He has received over $1,000,000 of external research funding in drug delivery and peptide research and has published numerous research articles with over 35 different undergraduate co-authors. He was named CASE Professor of the Year for the State of Wisconsin in 2001.

Mingfei Zhou
Professor Zhou received his B.S.and Ph.D degrees from Fudan University in 1990 and 1995, respectively. He was a postdoctoral fellow at the University of Virginia with professor Lester Andrews from 1997 to 1999. He then joined the chemistry department of Fudan University and became a professor of physical chemistry in 2000 and Cheung Kong Scholar in 2002. He is currently a member of the editorial advisory board of the Journal of Physical Chemistry.
Cindy Knight
Pat White

Huiwang Ai
Education
B.S. Tsinghua University, 2003
Ph.D. University of Alberta, 2008
Postdoctoral Fellow, The Scripps Research Institute, 2008-2011
Protein Engineering: Advancing Imaging, Therapeutics, and Diagnostics
The Ai lab specializes in protein engineering and employs interdisciplinary approaches, including biophysics, bioanalytical chemistry, chemical biology, synthetic biology, and optical imaging. One of our main areas of focus is the development of molecular biosensors that provide insights into cellular and organismal signaling. We employ state-of-the-art techniques such as directed evolution, fluorescence imaging, bioluminescence imaging, synthetic chemistry, genetic screening, and mass spectrometry to unravel complex biological pathways related to redox biology, metabolism, and neuronal activity. Furthermore, we explore the application of protein-based tools in live cells and rodent models to better understand pathophysiology and develop innovative therapies for conditions like diabetes, Alzheimer’s disease, and cancer. As an emerging direction, we are also working on the development of biosensors that can serve as self-wellness alert devices or diagnostics in resource-limited settings.
Recent publications:
H.W Yeh, and H-w. Ai*, “Development and Applications of Bioluminescent and Chemiluminescent Reporters and Biosensors”, Annu. Rev. Anal. Chem., 12: Doi: 10.1146/annurev- anchem-061318-115027.
H.W. Yeh, Y. Xiong, T. Wu, M. Chen, A. Ji, X. Li, and H-w. Ai*, “ATP-independent bioluminescent reporter variants to improve in vivo imaging”, ACS Chemical Biology, 2019, 14 (5): 959-965.
H.W. Yeh, O. Karmach, M. Martins-Green, and H-w. Ai*, “Red-shifted luciferase-luciferin pairs enhance bioluminescence in vitro and in vivo”, Nature Methods, 2017, 14: 971–974.
Y. Fan, and H-w. Ai*, “Monitoring thioredoxin redox with a genetically encoded red fluorescent biosensor”, Nat. Chem. Biol., 2017, 13: 1045-1052.
W. Ren, A. Ji, and H-w. Ai*, “Light Activation of Protein Splicing with a Photocaged Fast Intein”, J. Am. Chem. Soc., 2015, 137: 2155–2158.
A. Ji, W. Ren, and H-w. Ai*, “A Highly Efficient Oxidative Condensation Reaction for Selective Protein conjugation”, Chem. Commun., 2014, 50: 7469-7472
Z. Chen, W. Ren, Q.E. Wright and H-w. Ai*, “Genetically Encoded Fluorescent Probe for the Selective Detection of Peroxynitrite”, J. Am. Chem. Soc., 2013, 135: 14940-14943.
Awards and Honors
- The American Chemical Society 2017 Toxicology Young Investigators Award
- The Chinese-American Chemistry & Chemical Biology Professors Association (CAPA) Distinguished Junior Faculty Award, 2016
- National Science Foundation CAREER award, May 2014
- Hellman Fellows Award, The Hellman Fellows Fund, July 2013
- The Chinese American Faculty Association (CAFA) Robert T. Poe Faculty Development Award, February 2012

Alicia J. Frantz
Education
B.S. Ohio University, 2009
Ph.D. Ohio University, 2016
Alicia Frantz is an instructor for Organic Chemistry lecture. She earned a B.S. in Forensic Chemistry and a Ph.D. in Chemistry from Ohio University, Athens, Ohio. She is currently focusing on implementation of guided inquiry based learning in courses with large enrollment. Attending a large school herself, Alicia understands the importance for dedicating more of class time to group work and evidence-based techniques than the traditional lecturing. She is also updating her courses to reflect the growing diversity seen in the classroom, especially nontraditional and first generation students.
Alicia has taught general chemistry and organic chemistry at the University of Rio Grande and Kalamazoo College. She also spent a year as a medicinal chemistry intern at GlaxoSmithKline (GSK) in Research Triangle Park, North Carolina, synthesizing novel compounds in an effort to combat HIV/AIDS.

Earl Ashcraft, Ph.D.

Tao Huang
Tao received his B.S. from Wuhan University in China and earned his Ph.D. in medicinal chemistry from the University of Virginia, under the supervision of Professor Timothy Macdonald. His Ph.D. work focused on chemical biology study of Sphingosine 1-Phosphate (S1P) receptors and Sphingosine Kinases for immune modulation, which is directly relevant to human diseases such as autoimmune disorder and cancer. During his post-doc training in UVA’s School of Medicine, he extended his expertise into molecular imaging and nanotechnology for cancer diagnosis and therapy. In the Hsu lab, Tao will be working on immunotherapy for cancers.

Diane Dickie, Ph.D.
Dr. Diane Dickie joined the Chemistry Department in January 2018 as a Senior Scientist and X-Ray Crystallographer. Diane will also be working with the Materials Science Department. Her office is Room 103 in the Material Science Building. Diane received an honors B.Sc. from Mount Allison University in Sackville, New Brunswick, where she worked on the hydroboration of novel ketoenamines under the supervision of Prof. Stephen Westcott.
She earned her Ph.D. in inorganic chemistry at Simon Fraser University in Burnaby, British Columbia in Prof. Jason Clyburne’s lab, studying substituted m-terphenyl ligands in main group and transition metal chemistry. Her postdoctoral research on the activation of carbon dioxide with main group metal amides was conducted with Prof. Richard Kemp at the University of New Mexico and the Advanced Materials Laboratory of Sandia National Laboratories in Albuquerque, New Mexico.
While in New Mexico, Diane was heavily involved in Sandia’s award-winning elementary school science-outreach program “CSI: Dognapping”, and she was promoted to the position of Research Assistant Professor in UNM’s Chemistry department. Diane’s most recent position, before arriving at the University of Virginia, was as X-ray Diffraction Facility Director at Brandeis University in Waltham, Massachusetts.

Ryan Holland
Ryan Holland has joined the Department of Chemistry as a Research Associate in the Gunnoe Lab. Ryan obtained his B.S. in Chemistry at the University of Idaho, then went on to earn his Master’s Degree and Ph.D. in Chemistry at the University of California, San Diego. His graduate work consisted of studying the small molecule reactivity of Werner-type uranium complexes and the synthesis and characterization of stable metallacyclobutenes and vinylcarbenes. In the Gunnoe lab, Ryan will be engaged in the development of catalysts relevant to selective chemical oxidations, including the design, preparation and study of new catalysts.

Kinsuk Acharyya
Kinsuk Acharyya comes to the Chemistry Department from the Physical Research Laboratory in Navrangpura, Ahmedabad, India. He received his Ph.D. from the University of Calcutta in 2008. The thesis title was “Formation of Complex Molecules during Star Formation.” A part of this work was done in Leiden Observatory during his stay as a Greenberg Fellow. Additionally, he worked as a Research Scientist at the University of Virginia between March 2014 and December 2015. Kinsuk joins us as a Visiting Scholar in the lab of Professor Eric Herbst. Please welcome Kinsuk to the Chemistry Department!
Nathan Swami
Education
B.S. Indian Institute of Technology, Banaras Hindu University, Varanasi, India, 1991
M.S. University of British Columbia, Vancouver, 1993
Ph.D. University of Southern California, Los Angeles, 1998
Post-Doc Senior Scientist at Clinical MicroSensors Inc, a Caltech start-up focused on DNA sensors, 1999-2000
Principal Scientist at Motorola Labs, MEMS & Microfluidics 2000-2003
Biosystems often exhibit subpopulations with phenotypic heterogeneity, as part of their adaptation strategy to genetic and environmental influences. Stratifying this heterogeneity can lead to precision medicine-based approaches for disease diagnostics and for screening subjects towards advanced therapeutics. o quantitatively characterize this heterogeneity over multiple scales (cellular aggregates to cells to extracellular biomarkers), our research group is focused on developing microsystems for biophysical separation and bioanalytical detection. Some of the chief enablers in our group include: (1) soft imprint lithography-based patterning of soft materials as device platforms for enabling microfluidics and cell-laden hydrogel scaffolds; (2) electrochemical analysis in microfluidic and droplet systems for biomolecular sensing; and (3) label-free single-cell impedance and deformability-based cytometry and sorting, for quantifying the heterogeneity of stem cells, tumor cells, microbials and extracellular vesicles. In this manner, through coupling complex biological samples to microfluidic devices, circuits and signal analysis systems, we seek to impact emerging biomanufacturing approaches for regenerative medicine, as well as detection systems within point-of-care and resource-poor settings for personalizing medical diagnostics and therapeutics.
Representative Publications:
A. Rohani, J.H. Moore, Y-H. Su, V. Stagnaro†, C.A. Warren, N.S. Swami*, “Single-cell electro-phenotyping for rapid assessment of Clostridium difficile heterogeneity under vancomycin treatment at sub-MIC (minimum inhibitory concentration) levels”, Sensors & Actuators B: Chemical (2018), 276, 472-480. https://www.sciencedirect.com/science/article/pii/S0925400518315752
W. Varhue, L. Langman, M. Kelly-Goss, M. Lataillade, K. L. Brayman, S. Peirce-Cottler, N. S. Swami*, “Deformability-based microfluidic separation of pancreatic islets from exocrine acinar tissue for transplant applications”; Lab Chip (2017) 17, 3682 – 3691. https://pubs.rsc.org/en/content/articlelanding/2017/lc/c7lc00890b
Rohani, A.; Moore, J.; Kashatus, J.; Sesaki, H.; Kashatus, D.; Swami, N. S., “Label-free quantification of intracellular mitochondrial dynamics using dielectrophoresis”; Anal Chem (2017) 89 (11), pp 5757–5764. http://pubs.acs.org/doi/full/10.1021/acs.analchem.6b04666
A. Rohani, B. J. Sanghavi+, A. Salahi, K. –T. Liao+, C.-F. Chou, N.S. Swami*, “Frequency-selective electrokinetic enrichment of biomolecules in physiological media based on electrical double-layer polarization”; Nanoscale (2017), 9, 12124 – 12131. https://pubs.rsc.org/en/content/articlelanding/2017/nr/c7nr02376f/unauth#!divAbstract
Full List of Publications: https://scholar.google.com/citations?user=iS12HRMAAAAJ&hl=en

Ilse Cleeves
Education
B.S. Rice University, 2009
Ph.D. University of Michigan, Ann Arbor, 2015
Hubble Fellow at the Smithsonian Astrophysical Observatory in the Radio and Geoastronomy Division at Harvard-Smithsonian Center for Astrophysics and the Harvard Institute for Theory and Computation, 2015-2018
My research focuses on understanding the molecular and physical origins of planetary systems such as our own. By using clues from interstellar molecular emission, I study young planetary systems in formation around low-mass stars, i.e. protoplanetary disks: the very materials from which planets, comets, and other solar system bodies eventually form.
While I focus on the theoretical modeling of these systems, my work is guided by observational results from the Atacama Large Millimeter/Submillimeter Array, the Submillimeter Array as well as the Herschel Space Observatory. In addition to clues from the astronomical data, our group furthermore endeavors to connect all scales by incorporating our knowledge of the primitive solar nebula from the cometary and meteoritic record.
Recent Publications
"Constraining Gas-phase Carbon, Oxygen, and Nitrogen in the IM Lup Protoplanetary Disk," Cleeves, Oberg, Wilner, Huang, Loomis, Andrews, Guzman, 2018, ApJ, 865, 155.
"Variable H13CO+ Emission in the IM Lup Disk: X-ray Driven Time-Dependent Chemistry?," Cleeves, Bergin, Oberg, Andrews, Wilner, and Loomis, 2017, ApJL, 843, 1.
"Multiple Carbon Monoxide Snow-lines in Disks Sculpted by Radial Drift" Cleeves, 2016, ApJL, 816, 21.
"Indirect Detection of Forming Protoplanets via Chemical Asymmetries in Disks," Cleeves, Bergin, Harries, 2015, ApJ, 707, 2.
"The ancient heritage of water ice in the solar system," Cleeves, Bergin, Alexander, Du, Graninger, Oberg, and Harries, 2014, Science, 345, 1590.
Barat Venkataramany
Barat grew up in Ashland, OH, and graduated as the valedictorian from Ashland High School. He will be graduating from UVA with a Bachelor of Science in Chemistry (Specialization in Biochemistry) with ACS Certification and a minor in Psychology.
Upon beginning his studies at UVA, Barat joined the lab of Professor Wladek Minor in the Department of Molecular Physiology and Biological Physics (School of Medicine). Barat investigated serum albumin, the most abundant protein in mammalian blood plasma, and its interactions with non-steroidal anti-inflammatory drugs (NSAIDs). The goal of his project was to determine structures of mammalian albumins in complex with NSAIDs and understand how the modes of binding compare across species. Barat has been a co-author of three structures in the Protein Data Bank and of two publications (one article as the second author and a book chapter). He has two more manuscripts in writing.
Outside of the lab, Barat enjoys reading, watching films, listening to music, attempting to learn new languages (he speaks four), and playing the violin for First Year Players (for which he has played in five musical productions). He has also served a a TA for CHEM 4410 (Biological Chemistry I) for two semesters and for GNUR 5390 (Introduction to the U.S. Healthcare System) for three semesters. He also worked with the Undergraduate Research Network (serving as the Vice-Chair for one year) and volunteered at the UVA Medical Center through Madison House for six semesters. He also loves supporting his Cleveland and Columbus sports teams and following tennis, cricket, and golf, among many other sports.
Following graduation, Barat will attend medical school at the University of Toledo College of Medicine and Life Sciences.

Samuel Wachamo
Samuel grew up in Hawassa, Ethiopia until he moved to the U.S. and resided in Alexandria, VA in 2013. He attended T.C. Williams High School. The fact that Samuel has only been in the U.S. a few short years makes his success at UVA, and in general, that much more remarkable. Despite the myriad changes and challenges Samuel faced as his family resettled in northern Virginia, he graduated at the top of his class at T.C. Williams High School and received a full scholarship to the University of Virginia. He will be graduating from UVA with a Bachelor of Science in Chemistry (Specialization in Biochemistry) with ACS Certification and High Distinction.
Samuel got to know Professor Venton in her Analytical Chemistry Laboratory class, and he was attracted by her research program (Analytical Neurochemistry) which was in line with his aspirations. Venton lab is focused on the development of sensing and sampling techniques for the detection of new molecules in the brain. They aim to study the real-time release of many different neurotransmitters simultaneously to better understand healthy and diseased functioning of the brain. Samuel’s project in the Venton lab has a focus on electrochemical detection of Adenosine and ATP at carbon-fiber microelectrode (CFME) using Fast-Scan Cyclic Voltammetry (FSCV) and surfactant modification to promote selectivity. Adenosine and ATP are indispensable extracellular signaling molecules that are implicated in many disorders including neurodegenerative diseases. Given that the current techniques to measure the concentrations of Adenosine and ATP have slow time response, his project aims to develop a rapid analytical technique to discriminate and quantitate both Adenosine and ATP in real time. Samuel has successfully characterized the detection of Adenosine and ATP, and he is working on discriminating them.
An Echols scholar, Raven Scholar, and NSCS Scholar, Samuel is a member of the American Medical Student Association; Ethiopian-Eritrean Student Association (EESA at UVA); OneWay Intervarsity Christian Fellowship; Peer Advising Program in the Office of African American Affairs (OAAA) at UVA; and was a Madison House volunteer for Medical Services at UVA Hospital among many others. Outside of the lab, Samuel enjoys listening to Contemporary Christian songs, reading books (especially the Bible), running, playing soccer, playing piano, and learning new languages (he speaks English, Spanish, Amharic, Sidamic and a little French). He has been a small group leader at OneWay Intervarsity Christian Fellowship for the last three years where he plans and leads small group meetings and bonding activities. He has also been a peer advisor for the last two years; he serves as a mentor and advisor to six entering class of first-year and transfer students. Following graduation, Samuel will be doing a research internship (SRIP) at UVA School of Medicine, and eventually, he will be pursuing MD-PhD.

Elizabeth Lee
Education
Elizabeth is from Burke, Virginia and graduated from Lake Braddock Secondary School. She is graduating a year early with a Bachelor of Science in Chemistry with a specialization in biochemistry.
Elizabeth has been a member of Ken Hsu’s lab in the Department of Chemistry since September 2017. Her project has been based around expressing and purifying the mammalian enzyme, diacylglycerol kinase alpha (DGKα), in a bacterial system. DGKα has been recently recognized as a cancer therapy target due its important roles in regulating cell proliferation pathways. Elizabeth hopes that her project in the Hsu Lab will assist in the characterization of the enzyme in future proteomic experiments.
Outside of lab, Elizabeth played piccolo for the Cavalier Marching Band, worked with a company to train and develop AI software for colon cancer detection, and has been a TA for CHEM 2410 and CHEM 2321. After graduating, she will take a gap year to continue her project with DGK α and plans on pursuing an Md-PhD the following year.

Cliff Stains
Education
B.S. Millersville University, 2002
Ph.D. University of Arizona, 2008
NIH Ruth L. Kirschstein Postdoctoral Fellow, MIT, 2008-2011
Design and Application of Chemical Biology Tools for Studying Cellular Communication
Our laboratory conducts research at the interface of chemistry and biology by employing new chemical tools and design principles to better understand fundamental aspects of cellular communication. Our research program is centered on three areas involving: 1) synthesis and application of new fluorescent and luminescent bioprobes, 2) new methodologies for constructing designer signaling networks, and 3) new approaches to study protein folding mechanisms in living cells. The long-term goal of these projects is to provide insights into signaling processes associated with normal physiology as well as human diseases such as liver disease, diabetes, and cancer. These multidisciplinary efforts are supported by a combination of approaches including synthesis, protein design and evolution, bioanalytical chemistry, and molecular biology.
Selected Publications:
Zhou, X.; Fang, Y.; Lesiak, L. & Stains, C. I. A Phosphinate-Containing Fluorophore Capable of Selectively Inducing Apoptosis in Cancer Cells. ChemBioChem In Press.
Yuan, F.; Good, G. N.; Zhou, X. & Stains, C. I. Phosphinate-Containing Rhodol and Fluorescein Scaffolds for the Development of Bioprobes. Chem. Commun. 55, 5962-5965 (2019).
Zhou, X.; Lesiak, L.; Lai, R.; Beck, J. R.; Zhao, J.; Elowsky, C. G.; Li, H. & Stains, C. I. Chemoselective Alteration of Fluorophore Scaffolds as a Strategy for the Development of Ratiometric Chemodosimeters. Angew. Chem. Int. Ed. 56, 4197-4200 (2017).
Zhou, X.; Lai, R.; Beck, J. R.; Li, H. & Stains, C. I. Nebraska Red: A Phosphinate-Based Near-Infrared Fluorophore Scaffold for Chemical Biology Applications. Chem. Commun. 52, 12290-12293 (2016).
Beck, J. R.; Lawrence, A.; Tung, A.; Harris, E. N. & Stains, C. I. Interrogating Endogenous Phosphatase Activity with Rationally Designed Chemosensors. ACS Chem. Biol. 11, 284-290 (2016).
Zhao, J.; Nelson, T. J.; Vu, Q.; Truong, T. & Stains, C. I. Self-Assembling NanoLuc Luciferase Fragments as Probes for Protein Aggregation in Living Cells. ACS Chem. Biol. 11, 132-138 (2016).
Xu, B.; Zhou, X. & Stains, C. I. Supramolecular Assembly of an Evolved Miniprotein Host and Fluorogenic Guest Pair. J. Am. Chem. Soc. 137, 14252-14255 (2015).
Awards and Honors

Marilyne Stains
Education
B. S. Université des Sciences de Luminy, Marseille, France, 2001
M. S. Université Paul Sabatier, Toulouse, France, 2002
Ph. D. University of Arizona, 2007
Postdoctoral Research Assistant, University of Massachusetts, Boston, 2009-2011
Chemical Education Research: Enhancing Students’ Outcomes by Unpacking Postsecondary Instructors’ Practices
National initiatives to improve undergraduate science education build upon decades of research and development of effective instructional practices for science, technology, engineering, and mathematics (STEM) postsecondary classrooms. These evidence-based instructional practices (EBIPs) promote students’ conceptual understanding and attitudes toward STEM, with the greatest impacts observed among women and members of underrepresented groups. By improving retention rates and attracting a more diverse student population to the sciences, these practices can help address the national need to educate STEM-literate graduates and advance workforce development goals. The challenge is fostering their effective use on a national scale. Successful instructional reform requires STEM faculty engagement, yet we know little about them. We need to better understand faculty’s knowledge base for teaching, instructional practices and the relationship between the two as well as how these characteristics evolve under varying reform environments and departmental constraints. Results of this foundational research are critical to ensure the success of current and future national and local STEM instructional reform efforts. Our research group focuses on addressing this extensive gap in the literature. As discipline-based education researchers (DBER), we are specifically interested in:
- developing new methods to characterize instructional practices in STEM college classrooms,
- exploring how faculty and teaching assistants think about their teaching,
- identifying individual, departmental, and institutional factors that influence instructors' instructional decisions, and
- characterizing the impact of different types of pedagogical professional development programs.
Selected publications:
- Lane, A. K., McAlpin, J. D., Earl, B., Feola, S., Lewis, J. E., Mertens, K., Shadle, S. E., Skvoretz, J., Ziker, J. P., Couch, B. A., Prevost, L. B., & Stains, M.* (2020). Innovative teaching knowledge stays with users. Proceedings of the National Academy of Sciences, 202012372.
- Popova, M., Shi, L., Harshman, J., Kraft A., and Stains, M.* (2020) Untangling a complex relationship: Teaching beliefs and instructional practices of assistant chemistry faculty at research-intensive institutions, Chemistry Education Research and Practice, 21, 513-527 DOI: 10.1039/C9RP00217K
- Stains, M.*, Harshman, J., Barker, M.K., Chasteen, S.V., Cole, R., DeChenne-Peters, S.E., Eagan, M.K., Esson, J.M., Knight, J.K., Laski, F.A., Levis-Fitzgerald, M., Lee, C.J., Lo, S.M., McDonnell, L.M., McKay, T. A., Michelotti, N., Musgrove, A., Palmer, M.S., Plan, C.M., Rodela, T.M., Sanders, E.R., Schimpf, N.G., Schulte, P.M., Smith, M., Stetzer, M., Stewart, J., Van Valkenburgh, B., Vinson, E., Weir, L.K., Wendel, P.J., Wheeler, L.B., and Young, A.M. (2018) Anatomy of STEM Teaching in North American universities. Science, 359(6383), 1468 DOI: 10.1126/science.aap8892
- Harshman, J. and Stains, M.* (2017) A Review and Evaluation of the Internal Structure and Consistency of the Approaches to Teaching Inventory, International Journal of Science Education, 39(7), 918-936 DOI:10.1080/09500693.2017.1310411
- Stains, M.* and Vickrey, T. (2017) Fidelity of implementation: An overlooked yet critical construct to establish effectiveness of evidence-based instructional practices, CBE Life Sciences Education, 16(1), rm1 DOI: 10.1187/cbe.16-03-0113
- Lund, T.J. and Stains, M.* (2015) The Importance of Context: An Exploration of Factors Influencing the Adoption of Student-Centered Teaching among Chemistry, Biology, and Physics Faculty, International Journal of STEM Education, 2(13), DOI: 10.1186/s40594-015-0026-8
- Stains, M*, Pilarz, M., and Chakraverty, D. (2015) Short and Long-Term Impacts of the Cottrell Scholars Collaborative New Faculty Workshop, Journal of Chemical Education, 92(9), 1466-1476 DOI: 10.1021/acs.jchemed.5b00324
- Lund, T.J., Pilarz, M., Velasco, J.B., Chakraverty, D., Rosploch, K., Undersander, M., and Stains, M.* (2015) The Best of Both Worlds: Building on the COPUS and RTOP Observation Protocols to Easily and Reliably Measure Various Levels of Reformed Instructional Practices, CBE Life Sciences Education, 14(2), ar18 DOI:10.1187/cbe.14-10-0168
- Vickrey, T, Rosploch, K., Rahmanian, R., Pilarz, M. and Stains, M.* (2015) Research-based implementation of Peer Instruction: A literature review, CBE Life Sciences Education, 14(1) DOI: 10.1187/cbe.14-11-0198
See more (Google Scholar)

Jelena Samonina
Education
M.S. Organic Chemistry, Warsaw University, Poland, 2006
Ph.D. Organic Chemistry, Warsaw University, Poland, 2010
Visiting Research Fellow, National Institutes of Health, MD, 2012
Postdoctoral Research Associate, University of Virginia, Charlottesville, 2012 – 2014
Postdoctoral Fellow, Stanford University, CA, 2014 – 2016
Dr. Jelena Samonina joined the faculty of the UVA Chemistry Department in 2019, where her teaching interests include Organic Chemistry and Chemistry for the Health Sciences. She earned her M.S. and her Ph.D. in Organic Chemistry at Warsaw University. After two postdoctoral trainings at the University of Virginia and at Stanford University she joined the faculty of Washington and Lee University.
Dr. Samonina is passionate about teaching and she designs her courses to motivate and excite students through proactive learning. Her teaching style effectively engages the students and encourage them to develop into future thinkers, inventors, and researchers, whether it be in industry, academia, or public service.
Dr. Samonina’s research interests lie at the interface of Organic and Polymer Chemistry, Nanomaterials and Medicine, and include the development of new concepts in drug delivery, imaging and diagnostics.
Selected Publications
Sawyer C. W., Suffield B. H., Finnefrock A. C., Billings H. M., Uffelman E. S., Zoeller J. R., Dombrowski M. S., Delaney J.K., Dooley K. A., Mass J. L., Samonina J., Whitesell M. M., A John White Alexander Painting at the Virginia Museum of Fine Arts: A Comparison of Imaging Technologies for Resolving a Painting under Another Painting, Journal of American Institute for Concervation. 2019, 58, 37–53.
De Crisci A. G., Samonina-Kosicka J., Gieleciak R., Fleischauer M., Morris R. H., Waymouth R. M., Electrocatalytic Transfer Hydrogenation for Carbon Dioxide (CO2) Activation and Utilization” – 2018. Poster won first prize at the Connaught Global Challenge Symposium on CO2 Solution to Climate Change.
Butler, T.; Morris, W.; Samonina-Kosicka, J.; Fraser, C., Mechanochromic Luminescence and Aggregation Induced Emission of Dinaphthoylmethane β-Diketones and their Boronated Counterparts – ACS Applied Materials & Interfaces. 2016, 8, 1242–1251
Samonina-Kosicka, J.; Weitzel, D. H.; Hofmann, C. L.; Hendargo, H.; Hanna, G.; Dewhirst, M. W.; Palmer, G. M.; Fraser, C. L., Luminescent Difluoroboron β-Diketonate PEG-PLA Oxygen Nanosensors for Tumor Imaging. Macromolecular Rapid Communications 2015, 36, 694-699.
Butler, T.; Morris, W. A.; Samonina-Kosicka, J.; Fraser, C. L., Mechanochromic Luminescence and Aggregation Induced Emission for a Metal-Free Beta-Diketone. Chemical Communications 2015, 51, 3359-3362. - highlighted as "Noteworthy Chemistry" by ACS
DeRosa, C. A.; Samonina-Kosicka, J.; Fan, Z.; Hendargo, H. C.; Weitzel, D. H.; Palmer, G. M.; Fraser, C. L., Oxygen Sensing Difluoroboron Dinaphthoylmethane Polylactide. Macromolecules 2015, 48, 2967-2977.
Samonina-Kosicka, J.; Kańska, M., Synthesis of Selectively Labeled Histidine and its Methylderivatives with Deuterium, Tritium, and Carbon-14. Journal of Labelled Compounds and Radiopharmaceuticals 2013, 56, 317-320.

Ian Harrison
Education
B.S. Queen's University, 1981
Ph.D. University of Toronto, 1987
NSERC Postdoctoral Fellow, University of California, Berkeley, 1987-89
Surface Chemistry: Catalysis, Photochemistry, Reaction Kinetics & Dynamics
Catalysis is an essential technology supporting our way of life and contributes towards roughly 1/3 of the material GDP of the US economy. At the beginning of the 20th Century, the catalytic transformation of nitrogen on nanoscale, potassium promoted, iron catalysts to ammonia, and ultimately fertilizer, profoundly changed the human condition and currently supports an additional 2.4 billion people beyond what the Earth could otherwise sustain. In the 21st Century, the catalytic challenge will be to facilitate a rapid transition from a petroleum-based energy and chemical economy to a more generalized one based on natural gas, hydrogen, coal, biomass, and solar energy (photochemistry). Energy efficient chemical transformations, environmental protection, and green chemistry will continue to rely heavily on catalysis. Most industrially viable catalysis takes place on the surfaces of transition metal nanocrystallites dispersed on oxide supports. Our research focuses on understanding gas-surface reactions on simplified scientific model surfaces, namely, on single crystal surfaces. Recent progress includes the development of quantitative models for (i) the C-H bond activation of CH4 on metal surfaces that relates to the industrial production of H2 via natural gas reforming on metal nanocatalysts, and (ii) the chemical vapor deposition of Si on Si (100) by SiH4 that is central to Si homoepitaxy in microelectronics manufacturing. Current activities focus on exploring the thermal and photochemical reaction dynamics of catalytically important and energy-related small molecules, such as H2, CO2, CH4, alkanes, and alcohols, on transition metal surfaces. Our research typically employs ultrahigh vacuum surface analytical techniques (e.g., TPD, AES, XPS, RAIRS, STM, LEED) as well as some more specialized laser techniques (SFG, TOF) and/or microcanonical unimolecular rate theory. Our goal is to characterize the transition states of important catalytic reactions and to develop an improved understanding of how to design efficient & selective thermal and photochemically driven catalysts.
Recent Publications
Rice−Ramsperger−Kassel−Marcus Simulation of Hydrogen Dissociation on Cu(111): Addressing Dynamical Biases, Surface Temperature, and Tunneling. Donald SB and. Harrison I. J. Phys. Chem. C, 118, 320-337 (2014).
Methane dissociative chemisorption and detailed balance on Pt(111): Dynamical constraints and the modest influence of tunneling. Donald SB, Navin JK and Harrison I. J. Chem. Phys. 139, 214707 (2013).
Communication: angle-resolved thermal dissociative sticking of CH4 on Pt(111): further indication that rotation is a spectator to the gas-surface reaction dynamics. Navin JK, Donald SB, Tinney DG, Cushing GW, Harrison I. J Chem Phys. 136:061101 (2012).
Dynamically biased RRKM model of activated gas-surface reactivity: vibrational efficacy and rotation as a spectator in the dissociative chemisorption of CH4 on Pt(111). Donald SB, Harrison I. Phys Chem Chem Phys. 214:1784-95 (2012).
An effusive molecular beam technique for studies of polyatomic gas-surface reactivity and energy transfer. Cushing GW, Navin JK, Valadez L, Johánek V, Harrison I. Rev Sci Instrum. 82(4):044102 (2011).

W. Dean Harman
Education
B.S. Stanford University, 1983
Ph.D. Stanford University, 1987
Research Associate, Stanford University, 1988-89
For decades, the dearomatization of arenes has been recognized as a chemical transformation of fundamental importance. It provides the connection between this robust and abundant source of hydrocarbons and the alicyclic frameworks common to many biologically active products. Thus, dearomatization methods have become powerful tools for organic synthesis.
The chemical properties of arenes are influenced by their coordination to a transition metal. For example, complexes such as (η6-arene)Cr(CO)3+ and its cationic analogs (e.g., FeCp+, RuCp+, Mn(CO)3+) are susceptible to nucleophilic substitution or addition, ultimately leading to the formation of substituted arenes or cyclohexadienes, respectively. During the past three decades, the application of η6-arene complexes to organic synthesis has been widely explored.
In the Harman Research Group, an alternative approach to the dearomatization of arenes has been developed in which the aromatic system may be activated toward organic transformations by η2-coordination. Here, the metal-arene bond is stabilized primarily by the interaction of filled metal dπorbitals with the π* system of the arene, an interaction having two important consequences for the activation of the aromatic ring. Through πbackbonding, the aromatic π system becomes more electron-rich, similar to what is observed for organic arenes bearing electron-donating groups. Additionally, structural data for complexes containing η2-coordinated aromatic rings show significant distortions in the bond lengths of the ring consistent with a localization of πelectron density. Together, these effects activate η2-bound aromatic systems toward electrophilic rather than nucleophilic addition. When the π base binds an aromatic heterocycle such as a pyridine, furan or pyrrole, novel tandem addition reactions and cycloaddition reactions become possible.
Of the handful of transition metal systems that are known to form stable η2 complexes with aromatic molecules, only d6 octahedral metal complexes have been shown to enhance the reactivity of the aromatic ligand toward electrophiles to date. For nearly a decade, despite our best efforts, this mode of arene activation was known only for the pentaammineosmium(II) system. However, in the past few years a new generation of dearomatization agents have been developed in our group based on a careful matching of the d5/d6 reduction potential of rhenium(I), tungsten(0), and molybdenum(0) complexes with that of pentaammineosmium(II).
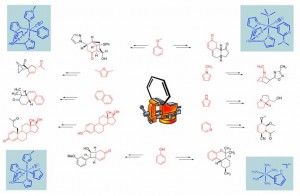
Recent Publications
Group 6 Dihapto-Coordinate Dearomatization Agents for Organic Synthesis Liebov, B. K.; Harman, W. D., Chem. Rev. 2017, 117, 13721-13755.
Molybdenum-Promoted Synthesis of Isoquinuclidines with Bridgehead CF3 Groups Wilde, J. H.; Smith, J. A.; Dickie, D. A.; Harman, W. D., J. Am. Chem. Soc. 2019, 141, 18890-18899.
Highly Functionalized Cyclohexenes Derived from Benzene: Sequential Tandem Addition Reactions Promoted by Tungsten Wilson, K. B.; Smith, J. A.; Nedzbala, H. S.; Pert, E. K.; Dakermanji, S. J.; Dickie, D. A.; Harman, W. D., J. Org. Chem. 2019, 84, 6094-6116.
Preparation of Cyclohexene Isotopologues and Stereoisotopomers from Benzene Smith, J. A.; Wilson, K. B.; Sonstrom, R. E.; Kelleher, P. J.; Welch, K. D.; Pert, E. K.; Westendorff, K. S.; Dickie, D. A.; Wang, X.; Pate, B. H.; Harman, W. D., Nature 2020, 581, 288-293.
Experiments and Direct Dynamics Simulations that Probe η2-Arene/Aryl-Hydride Equilibria of Tungsten Benzene Complexes Smith, J. A.; Schouten, A.; Wilde, J. H.; Westendorff, K. S.; Dickie, D. A.; Ess, D. H.; Harman, W. D., Journal of the American Chemical Society 2020, 142, 38, 16437–16454.

Meagan Belcher Dufrisne
Meagan is a Postdoctoral Fellow in the laboratory of Linda Columbus. Her research aims to identify the molecular determinants of the interactions between neisserial Opa proteins and their human receptors in vitro, in order to gain a better understanding of pathogen-host interactions. She obtained a B.S. in Biochemistry and a B.A. in Discrete Mathematics from the University of Nevada, Reno in 2011. Meagan received her PhD in 2018 from Columbia University, where she worked in the laboratory of Filippo Mancia using structural biology techniques such as X-ray crystallography and cryo-EM to study integral membrane enzymes involved in biosynthesis of the bacterial membrane.
Pargat Singh
Pargat did his Ph.D. from CSIR-Institute of Microbial Technology in collaboration from Panjab University, Chandigarh (India). His Ph.D. work focused on development of low cost nanomaterial-based detection strategies for pathogens in water and typhoidal antigens in blood serum. Here at UVA he is associated with the Pompano Lab for detection of signaling proteins in live tissues.

Mehrdad Shadmehr
Mehrdad Shadmehr received his Ph.D. from the University of Arizona. His Ph.D. project was mainly about the design and synthesis of fluorogenic triazabutadiene probes for the labeling of electron-rich aromatic systems. M.S from Southern Illinois University Edwardsville, where he worked on the synthesis of bioactive fluorinated heterocyclic small molecules. B.S from Tehran University. He started his career as a postdoctoral research associate at the University of Nebraska-Lincoln in Professor Cliff Stains research group in 2018. He is currently a postdoctoral research associate at the University of Virginia working on design and synthesis of organic small molecule fluorogenic fluorophores, and design and synthesis of second window near-infrared fluorophores.

Eduard Dumitrescu
Eduard graduated with a Ph.D. from Clarkson University in May 2019 under the supervision of Dr. Silvana Andreescu. His work there was focused on the use of zebrafish embryos as a biological model (1) for toxicological assessment of nanomaterials and chemical additives employed in industrial and consumer applications, and (2) for in vivo electrochemical monitoring of biomarkers related to oxidative stress and neurotransmission. Eduard joined Dr. Venton’s lab in June 2019 where he currently works on electrochemical detection of neurotransmitters in Drosophila neurological disorder models.

Nathan Coutard
Nathan Coutard graduated in 2015 from Université Pierre et Marie Curie in Paris. His end of masters internship was in Pr. Kylie Vincent’s lab at University of Oxford where he studied the regulatory hydrogenase from R. eutropha using in operando spectro-electrochemistry. He then joined the Artero group at Université Grenoble Alpes for a PhD project that aimed to optimize and integrate bio-inspired catalysts in a functional proton exchange membrane fuel cell. In the Gunnoe group, he now works on light alkane oxidation.

Yuan Fang
Yuan received her Ph.D. in Applied Chemistry from Nanjing Tech University, China. Her Ph.D. work focused on synthesis and application of rhodamine-based small molecular chemosensors for sensing heavy toxic metal ions. She joined Professor Cliff Stains group as a research associate in 2017, currently, she is working on developing novel near-infrared fluorophore scaffolds as bioimaging reagents for proteins, small molecules, and post-translational modifications.

Ranjit Mondol
I am from West Bengal, India. In 2012, I obtained my bachelor’s degree in chemistry at Ramakrishna Mission Vidyamandira (under Calcutta University), Belur Math, West Bengal, India. In 2014, I earned my master”s degree in chemistry at Indian Institute of Technology (IIT), Kanpur, Uttarpradesh, India. Then, in 2015, I moved to Groningen, Netherlands and joined as a graduate researcher in the Prof. Dr. Edwin Otten’s group at the University of Groningen, Netherlands where I studied extensive synthesis and characterization of redox-active ligand frameworks and molecular metal complexes with detailed analysis of electrochemical properties aiming towards development of catalysts with abundant and cheap main group elements based complexes. In 2019, I obtained my doctorate degree in chemistry at the University of Groningen under the supervision of Prof. Dr. Edwin Otten. Then, from January, 2020, I have been appointed as a postdoctoral research associate in the group of Dr. Robert J. Gilliard at the University of Virginia, USA. At Prof. Gilliard’s lab my research focuses on chemical synthesis and the development of methods to incorporate p-block elements into conjugated small molecules and polymers for optoelectronic applications (e.g., polyaromatic hydrocarbons, heterocycles, metallacycles).
Marcos Pires
Education
B.S. Ithaca College, 2003
Ph.D. Purdue University, 2009
NIH Postdoctoral Fellow, University of Pennsylvania, 2009-2011
The Pires lab uses synthetic chemistry as a platform to construct cell wall analogs that metabolically label live bacteria and mimic key aspects of cell wall architecture. Through this work, the interrogation of cell wall remodeling and processing in pathogenic bacteria will guide the design of next-generation antibiotics that circumvent resistance mechanisms. Moreover, we are working to establish the fundamental framework of a non-traditional antibiotic therapy based on the specific recruitment of components of the immune cells to target the destruction of pathogenic bacteria.
Every year in the United States, over two million people are afflicted with bacterial infections resistant to FDA-approved antibiotics. In order to counter the rapid rise in drug-resistance in bacteria, new drug targets and diagnostic tests are urgently needed. The bacterial cell wall has proven to be a rich source of antibiotic drug discovery. However, there are fundamental aspects of bacterial cell wall assembly and its interaction with the host organism that are yet to be fully elucidated.
Bacterial Cell Wall Probes – Chemical Microbiology
The Pires laboratory will leverage the laboratory expertise in building synthetic bacterial cell wall mimics to reveal key aspects of peptidoglycan recognition and cell wall remodeling. We anticipate that interrogation of cell wall remodeling and processing in pathogenic bacteria will guide the design of next-generation antibiotics that circumvent resistance mechanisms. Our work has yielded synthetic cell wall mimics that reveal how bacterial cell wall building blocks regulate surface remodeling and biosynthesis.
Synthetic Immunotherapeutics Against Bacterial Pathogens
We are developing unique antimicrobial therapeutic strategies based on a specific modulation of the immune response to combat bacterial infections. We have established novel methods to re-engage components of the immune system to induce a targeted immunological response to the site of infection. The goal is to assemble and evaluate agents that induce the recruitment of antibodies or the direct engagement with human immune cells. This new class of agents will constitute a way of mobilizing the immune system to target poorly immunogenic bacterial pathogens.
RECENT PUBLICATIONS
Apostolos AJ, Kelly J, Ongwae G., Pires M."Structure Activity Relationship of the Murein Peptide in Sortase A mediated Ligation from Staphylococcus aureus" (2021), in preparation.
Ferraro NJ, Pires M. "Transposon Screen of Surface Accessibility in Staphylococcus aureus" (2021), submitted.
Apostolos AJ, Yehiya TK, Silva JR, Lameira J, Alves MC, Siegrist MS, Pires M. "Metabolic Processing of Selenium-based Bioisostere of meso-diaminopimelic Acid in Live Bacteria" (2021), submitted.
Ongwae G, Chordia M, Cawley JL, Dalesandro BE, Wittenberg NJ, Pires M. "Targeting of Pseudomonas aeruginosa Cell Surface via GP12, an Escherichia coli Specific Bacteriophage" Scientific Reports (2021), accepted.
Apostolos AJ, Pires M. "Impact of Crossbridge Structure on Peptidoglycan Crosslinking: A Synthetic Stem Peptide Approach" Methods in Enzymology (2021), accepted.
Sikorski EL, Wehr J, Ferraro NJ, Pires M, Thévenin D. "Selective Display of a Chemoattractant Agonist on Cancer Cells Activates the Formyl Peptide Receptor 1 on Immune Cells" ChemBioChem (2021), accepted.
Ferraro NJ, Kim S, Im W, Pires M. "Systematic Assessment of Accessibility to the Surface of Staphylococcus aureus." ACS Chemical Biology (2021) 16, 2527-2536.
Apostolos AJ, Ferraro NJ, Dalesandro BE, Pires M. "SaccuFlow: A High-Throughput Analysis Platform to Investigate Bacterial Cell Wall Interactions." ACS Infectious Diseases (2021) 7, 2483-2491.
Wehr J, Sikorski EL, Bloch E, Feigman MS, Ferraro NJ, Baybutt TR, Snook AE, Pires M, Thévenin D. "pH-Dependent Grafting of Cancer Cells with Antigenic Epitopes Promotes Selective Antibody-Mediated Cytotoxicity. Journal of Medicinal Chemistry (2020), 63, 3713-3722.
Dalesandro BE, Pires M. “Induction of Induction of Endogenous Antibody Recruitment to the Surface of the Pathogen Enterococcus faecium.” ACS Infectious Diseases (2020), 7, 1116-1125.
Apostolos AJ, Nelson J, Pires M. “Facile Synthesis and Metabolic Incorporation of m-DAP Bioisosteres Into Cell Walls of Live Bacteria.” ACS Chemical Biology (2020), 15, 2966-2975.
Apostolos AJ, Pidgeon SE, Pires M. “Remodeling of Cross-bridges Controls Peptidoglycan Cross-linking Levels in Bacterial Cell Walls.” ACS Chemical Biology (2020), 15,1261-1267.
Ongwae GM, Morrison KR, Allen RA, Kim S, Im W, Wuest WM, Pires M. “Broadening Activity of Polymyxin by Quaternary Ammonium Grafting.” ACS Infectious Diseases (2020) 6, 1427-1435.
Wehr J, Sikorski EL, Bloch E, Feigman MS, Ferraro NJ, Baybutt TR, Snook AE, Pires M, Thévenin D. “pH-Dependent Grafting of Cancer Cells With Antigenic Epitopes Promotes Selective Antibody-Mediated Cytotoxicity.” Journal of Medicinal Chemistry (2020) 63, 3713-3722.
Wang L, Jakob S, Wang H, Apostolos A, Pires M, Xu XG. “Generalized Heterodyne Configurations for Photoinduced Force Microscopy.” Analytical Chemistry (2019) 91, 13251-13259.
Pidgeon S, Apostolos A, Nelson J, Shaku M, Rimal B, Islam M, Crick C, Kim J, Pavelka S, Kana D, Pires M. “L,D-Transpeptidase Specific Probe Reveals Spatial Activity of Peptidoglycan Cross-Linking.” ACS Chemical Biology (2019) 14, 2185-2196.

Lucas Frye
Lucas is from Severna Park, Maryland, and graduated from Severna Park High School. He is graduating with a Bachelor of Science in Chemistry, with a specialization in environmental chemistry.
Lucas has been a member of Brent Gunnoe's lab in the Department of Chemistry since May 2018. His project has focused on the development of new methods for the synthesis of stilbenes through Rh-catalyzed aerobic alkenylation of arenes. Stilbenes are used as dyes, pharmaceuticals, and precursors to electronic materials. Lucas hopes that his work will provide new tools for chemists to produce these useful compounds more efficiently and with less waste.
Outside of lab, Lucas has represented the American Chemical Society at the United Nations Climate Change Conference and helped lead the Students on Climate Change project, with the aim of improving climate literacy among students and the general public. He has also been a teaching assistant for CHEM 1411 and CHEM 1421. Lucas is a member of Alpha Chi Sigma, the professional chemistry fraternity, and Amuse Bouche, UVA's long-form improvisational comedy troupe. After graduating, he will attend graduate school to pursue a Ph.D. in inorganic chemistry.
Eric Wang
Eric is from Boston Massachusetts but graduated from Oakton high school in Fairfax Virginia. He is graduating with a Bachelor of Science in Chemistry with a specialization in biochemistry and a Bachelor of Art in Computer Science with distinguished major.
Eric joined Prof. John Fu’s lab in the Department of Pharmacology since his second year. His projects oriented around studying the mechanism and variants of Ciliogenesis associated kinase 1 (CILK1), with several main themes including the function of CILK1 c-terminal domain (CTD), the cellular effects of juvenile myoclonic epilepsy (JVM) variants of CILK1, and the
importance of Kif3a protein as a direct substrate of CILK1 in mouse models. Eric’s work with Dr. Fu has resulted in two co-first author publications and one more to be submitted.
For his CS DMP with Prof. Yanjun Qi and QData lab, Eric studied the application of deep learning on CRISPR off-target prediction, protein folding and interaction, and epigenetic. His thesis discussed a novel dual-NeuralNet model that uses DNA sequence with epigenetic annotations and Hi-C map to rank variants based on their likelihood of causing coronary artery disease.
Outside of lab, Eric volunteered for the Department of Cardiology at the UVa hospital. He was also a committee member for Uva FoodAssist, a club that bridges food waste and food insecurity by gathering and donating food. Eric was a member of Team Virginia at iGEM2017.
Upon graduation, Eric will apply to MD-PhD programs and spend his bridge year in Charlottesville as a lab technician for Dr. Lawrence Lum.
Tina Chai
Tina Chai is from Falls Church, Virginia and graduated from McLean High School. She is graduating from U.Va. with a Bachelor of Science in Chemistry with a specialization in Biochemistry and a Bachelor of Arts in English.
Tina has been a member of Dr. Robin Felder’s lab in the Department of Pathology since January 2018. Her independent project investigates the relationship between breast cancer and hypertension by determining an overlapping protein signaling pathway specifically related to three proteins: CAV1, D1R, and GRK4. She has also previously conducted breast cancer research at the MD Anderson Cancer Center.
Outside of lab, Tina enjoys writing poetry and busking at the Downtown Mall. She served as the editor for the Health and Science section of The Cavalier Daily and a teaching assistant for CHEM 4410, CHEM 1410/20, and CHEM 1411/21. She also enjoys furthering interdisciplinary thought and dialogue between science and literature through The Bird-Feeder Project, Whistle Words writing workshops, and a class she created and teaches, INST 1550 - Poetry and Healing: Understanding Illness. After graduation, Tina will be attending a writing program at Johns Hopkins University and plans to apply to medical school.
Colton Sheehan
Colton Sheehan is from Farmville, Virginia and graduated from Prince Edward County High School. He will be graduating with a Bachelor of Science in Chemistry with a specialization in Materials Science, as well as a Bachelor of Arts in Physics and a Minor in Math.
Colton has been a member of Dr. Sen Zhang's lab since his second year. His work focuses on the development of novel synthesis methods for various nanomaterials. The functionality of these nanomaterials as catalysts of energy conversion reactions using electrochemical analysis. Colton hopes his research will help propel society to a sustainable energy future.
Outside of lab, Colton is involved with volunteering through Madison House and a local nonprofit, called Code Ana. In his free time, he likes to jog, spend time with friends, and watch Netflix. After graduation, he will attend graduate school in pursuit of a PhD in Materials Science.
Andrew Chung
As a student at the University of Virginia, Andrew Chung started his undergraduate journey attending Professor Walter D. Harman’s morning lecture, Principles of Chemical Structure. Learning from Professor Harman was an edifying experience that allowed for viewing chemistry from different perspectives. Furthermore, one of the biggest lessons taken away from Professor Harman was seeing things from a beginner’s point of view. From how this idea was simply put, Andrew was fascinated with a lifelong pursuit in not only uncovering new mysteries and curiosities but also with learning how to learn and finding ways to convey new ideas for others to better understand how to advance the well-being of those around us today and future generations. As an Echols Scholar, Andrew was invited by Professor Harman to explore organometallic dearomatization agents after finishing his class in first year and discussed potential directions for undergraduate research with Professor Harman, in which Andrew started in his second year at the UVa.
As an undergraduate researcher, Andrew collaborated with Professor Harman and three graduate students with the goal of optimizing features of synthetic pathways of important coordinatively saturated organometallic dearomatization agents using W(I) and Mo(I) transition metal centers. Through his work, Andrew had a full experience of an independent researcher by testing his hypotheses and redesigning experiments. From having a broad coverage of experimenting with different initiatives in the lab, Andrew used this well of experience to discover how to improve the reduction of W(I) and Mo(I) dearomatization agents using a magnesium reducing agent instead of a pyrophoric sodium reducing agent, which presented advantages of a more suitable chemical for both lab and industrial purposes. Working as an independent researcher, Andrew was able to publish his work on a collaborative journal article about the application of magnesium reductions on the second-row d6-transition Mo(I) metal center in ACS Catalysis.
Growing up in Yorktown, Virginia, Andrew developed his aspiration to practice medicine and pursue his interests in chemistry. Andrew was accepted into the University of Virginia’s Echols Scholar program in his senior year at Tabb High School. In addition to his research in the Chemistry Department, Andrew also serves as the head teaching assistant of the Accelerated Organic Chemistry Series at UVa and is a member of Alpha Chi Sigma, the professional co-ed chemistry fraternity at UVa. As part of his undergraduate experience, Andrew has worked as a Team Leader for the Youth Volunteer Corps Hampton Roads’ Summer of Service volunteer programs and as a Child Life Volunteer for patients at the Children’s Hospital of the King’s Daughters, a regional children’s hospital in Norfolk, Virginia. In his spare time, Andrew loves to stay active and joined the Barbell Club to push himself with new workouts and a great community of fellow exercise enthusiasts. Andrew also has written a draft of a part-autobiographical musical, The Languages of Love, and has performed at open mics as a member of Flux Poetry & Spoken Word at UVa with other peers who share a passion for bringing people together through our greatest tool, communication. As a musician, Andrew also taught himself to play the guitar, learned to sing, and continues to play the piano and trumpet when he can to explore different genres and tastes in music. Andrew is also a member of the UVa Chapter of the National Society of Collegiate Scholars, the national academic collegiate honor society. Completing his curriculum in the Department of Chemistry at UVa with his Distinguished Majors Program Thesis, Andrew will graduate this spring with a Bachelor’s in Science degree in Chemistry, with a Specialization in Biochemistry and with American Chemical Society Certification.

Robert Fisher
Robert grew up in McLean, VA and graduated from Trinity Christian School in Fairfax. He will be graduating from UVA with a Bachelor of Science in Chemistry with Specialization in Biochemistry, with ACS certification and a minor in Psychology.
He joined the lab of Dr. Kevin Lehmann in the Department of Chemistry in the fall of 2017. He has researched the interaction of mercury vapor with light at 405 nm and 254 nm in an attempt to describe the formation of a metastable state. The goal of this project is to test the validity of a kinetic model that would allow mercury to be detected with high fidelity in trace amounts using 405 nm lasers.
Robert enjoys writing and playing tabletop games with his friends. He worked as a volunteer at the UVA Battle Building through Madison House Medical Services for his entire time at UVA. He also recently regained an interest in running.
After graduating, Robert will take a gap year in order to take the MCAT, apply to medical school, and work a job.

Chris Pede
Chris Pede is from Lynchburg, Virginia and was homeschooled, meaning he graduated high school as both valedictorian and bottom of his class. He's graduating from UVA with a Bachelor of Arts in Chemistry.
Chris became interested in astronomy after taking a class on Black Holes his second year. Chris worked with Dr. Ilse Cleeves on tracing chemical asymmetries in rotational emission around a young stellar object to infer the presence of a planet. Detecting deviations from the expected rotational emission helps us understand the chemical and physical effects a planet would have in a protoplanetary disk.
Outside of lab, Chris is a teaching assistant for physical and organic chemistry, a personal trainer at the AFC, and a volunteer with the Sexual Assault Resource Agency, an organization dedicated to ending sexual violence. He is a dedicated tattoo aficionado whose enthusiasm for art is not shared by his mother. Chris spends what little free time he has left cooking, lifting weights, and playing basketball. Next year, Chris will be attending an inferior basketball school and getting his Masters Degree in Biomedical Sciences at Duke, while applying to medical school for the following year.

Mehron Kouhestani
Mehron is from Virginia Beach, Virginia and graduated from Ocean Lakes High School. He is graduating with a Bachelor of Science in Chemistry with a specialization in biochemistry.
Mehron has been a member of the Hockensmith lab in the Department of Biochemistry and Molecular Genetics since June 2018. His project has been based around identifying the structure of an Active DNA-dependent ATPase A Domain inhibitor (ADAADi) as a model for SNF2 Chromatin Remodeling enzymes that play a role in cancer proliferation. ADAADi has the potential to be the next best cancer treatment as it can inhibit chromatin remodeling to prevent transcription. Mehron hopes that his project in the Hockensmith Lab will assist in the finding the structure of the unknown compound in future experiments.
Outside of lab, Mehron was the President of the Persian Cultural Society, Office of Health Promotion Intern, Peer Health Educator, Secretary of Neuroscience Enthusiasts Club, Outreach Chair of Charlottesville Alliance for Refugees, a volunteer for Computers 4 Kids, and has been a TA for CHEM 1411. After graduating, he will take two gap years to pursue his interests and prepare for medical school.

Sean Lee
Sean has been a member of Dr. Liheng Cai’s lab in the Department of Materials Science and Engineering since August 2018. His independent project investigates the potential use of the PS-bbPDMS-PS triblock copolymer in double emulsion fabrication. The double emulsion serves as a platform technology for which it could be used in various settings such as drug delivery, cellular research, etc.
Outside of lab, Sean enjoys listening to music and compiling playlists for different occasions. Sean served as a teaching assistant for CHEM 3410/3420 and MSE 3050 and worked as a bartender at a local restaurant on the weekends. Sean is fascinated with the structure-property relationship of a polymer and will be pursuing a PhD in chemistry to expand his understanding.

Sadeechya Gurung
Sadeechya Gurung is from Falls Church, Virginia, graduating from Justice High School in 2016. She is graduating from U.Va with a Bachelor of Science in Chemistry with Specialization in Biochemistry and a Minor in Religious Studies.
Since January 2019, Sadeechya has worked with Dr. Ken Hsu of the Hsu Lab in the Department of Chemistry. Her research has contributed to efforts in crystallizing the first full-length mammalian Diacylglycerol Kinase α (DGKα) by expression and purification from a bacterial system. This enzyme plays a crucial role in modulating diacylglycerol and phosphatidic acid levels in cells, two lipid second messengers implicated in the growth of many types of cancer cells. Thus, structural analysis of DGKα will greatly aid efforts in developing novel immunotherapies through drug discovery. She has also worked to express and purify Serine/Arginine Splicing Factor 1 RNA Recognition Motif 1 (SRSF1-RRM1) for Nuclear magnetic resonance (NMR) studies of its binding capacities. SRSF1 is a protein with oncogenic potential as it plays an important role in alternative splicing which could contribute to tumorigenesis.
Aside from research, Sadeechya worked as a teaching assistant for CHEM 2411/2421 (Organic Chemistry Lab for Chem Majors) for two years and CHEM 2311/2321. She volunteered as a mentor at Computers 4 Kids and in Madison House programs such as Pet Pals at the CASPCA and Adopt-a-Grandparent at The Heritage Inn. She also served as an officer for Nepali Student Association to help cultivate the Nepali community at U.Va. In her free time, Sadeechya enjoys drawing, painting, and avidly bullet journaling. After graduation, she plans to work for a few years before furthering her education in science.
Merly Konathapally
Merly Konathapally is from Virginia Beach, Virginia and graduated from Ocean Lakes High School. She is graduating from U.Va. with a Bachelor of Science in Chemistry with a Specialization in Biochemistry and a minor in Psychology.
Merly joined Dr. Robert Grainger’s lab in the Department of Biology during fall 2018. Her project was focused on developing an understanding of the six3 mutation in Xenopus and the role of transcription factors in signaling development at embryonic stages. Her research aimed to further understand the role of six3 in signaling eye development by using mRNA probes to indicate downstream targets. She researched lens development through a variety of methods, including in situ hybridization, microscopic imaging, and PCR. Her work also analyzed the use of mRNA injections to rescue lens phenotypes.
In addition to research, Merly worked as a medical scribe in the UVA Emergency Department and as a TA for the organic chemistry lab. She also served as the Senior Resident at Brown College and volunteered with the daycare program at Madison House. She also spent a summer conducting research on water quality in South Africa through a grant from UVA’s Center for Global Health. After graduating, Merly will be completing a one-year program for a Master of Public Health (MPH) at UVA.

Luke Cavanah
Luke is a fourth-year undergraduate pre-medical student at UVA, majoring in chemistry (with specialization in biochemistry) and psychology. Aside from classes, he works as a certified nursing assistant and is conducting research under Prof. Morris’s guidance in social neuroscience research. Luke’s independent research project will be extending previously conducted research on a study examining the effects of valence and concurrent task difficulty on the late-positive potential (LPP), where the stimuli presented were words. Due to the visual nature of most emotional stimuli prevalent in day-to-day life and the importance of the LPP in memory encoding and storage, he is interesting in examining: How does the difficulty of a concurrent task alter the LPP corresponding to the processing of emotional images of varying levels of arousal and different valences? The LPP is a type of event-related potential (ERP), which is implicated in a variety of long-term cognitive processes and is notable for its unique stability across repeated stimulus presentations. ERPs refer to small but distinct changes in the voltage recorded by an electrode in electroencephalography, which arise due to specific events or stimuli.

Chase Amos
“The Role of Plasma Membrane Order in Insulin Vesicle Exocytosis”
Insulin is a hormone that helps the body regulate glucose levels. When there is high glucose in the blood, beta cells in the pancreas release insulin via a process known as exocytosis. Biological cells are separated from their environment by a membrane that consists of lipids and proteins. Growing evidence shows that lipids in the membrane intimately participate in the molecular machinery that drives and controls exocytosis. In my research, I address the contribution of the balance between lipids with saturated and unsaturated fatty acyl chains to exocytosis of insulin by beta cells. Our data indicates that regulated exocytosis depends on a balance between membrane fusion promoting unsaturated fatty acyl chains and membrane fusion inhibiting saturated fatty acyl chains. I am now further exploring the molecular origins for the lipid influence in insulin exocytosis in Dr. Lukas Tamm’s lab in the UVA Molecular Physiology & Biophysics Department, with mentorship within the lab from Dr. Volker Kiessling.
I am pursuing my B.Sc. in biochemistry at UVA (class of 2021). After completing my bachelor’s, I hope to go onto graduate school for a PhD. My ultimate goal is to become a professor and scientist in academia, studying membrane biology in the lab. I hope to advance our understanding of diseases related to membrane biology.

Julia Dressel
Rising carbon emissions from industrial sources have cascading impacts on the environment, but it is challenging to mitigate climate concerns while meeting current energy demands. An attractive solution would use renewable energy to electrochemically reduce CO2 into valuable building blocks for chemicals and fuels or to produce hydrogen. Because these reactions are energy intensive, catalysts have been developed to make these processes more efficient. In an effort to improve upon known catalysts, my Harrison research project seeks to investigate a new molecular nickel complex as an electrocatalyst for these energy relevant reactions using several electrochemical, spectroscopic, and computational techniques.
Julia Dressel is a third-year chemistry and environmental science major from Clifton, VA. She is thankful for her faculty mentor, Dr. Charles W. Machan, her graduate student mentor, Shelby Hooe, and the rest of the Machan laboratory for her great research experiences.

Zhiwen Xu
I am Zhiwen Xu, currently a rising second-year planning on majoring in biochemistry and studio art major on a pre-med track.
I have loved science as a child starting with my first science fair project, "Temperature and Bee" in fifth grade. I continued to do science projects throughout high school, where I began to develop an interest in gene interaction within human bodies and was fascinated to find solutions to lower the occurrence of inflammatory disease. In one of the projects, I discovered that dandelion extracts could suppress colonies of E.coli, in which the project was selected to participate in the International Science and Engineering Fair in 2017. At UVA, I continued my pursuit of science. I joined Professor Mete Civelek's lab in the biomedical engineering department, starting my first year of fall semester 2019.
Along with my mentors, my current research focuses on investigating how KLF14 functions differently in female and male adipocyte cells by comparing the KLF14 gene expression between the knockout and wild-type mice within the different sexes. As the prevalence of type 2 diabetes mellitus (T2D) increases rapidly worldwide, with 642 million cases projected to occur by 2040. However, among the increasing population that has T2D, the susceptibility of disease differs between females and males, partly due to the different patterns of body composition and fat distribution. Thus, it is crucial in understanding the sex-specific influences on metabolism that involves the genetic regulation of gene expression in adipose tissues, which could lead to the development of new customizable therapies for individuals to treat T2D more effectively. I am deeply thankful for my mentors, Dr. Mete Civelek, Dr. Qianyi Yang, James Hinkle, and the rest of the lab members.
Outside the lab, I love to draw and design using mixed media. Some of my works were published in magazines recently, such as Scratch Zine and Gilerzine. I am also interested in fashion, so I was part of the Fashion for A Cause fashion show fall of 2019 as a stylist, where many of the donations go toward the community. I also have founded a design/product business called MoonPrint that started a few years ago, right now, I am planning to continue and grow MoonPrint this summer. Moreover, I am currently part of the MindBody at UVA executive team as the marketing and design officer. I am always curious about the world and love to try and explore new things. In my free time, I love hanging out with friends, dancing, singing or playing music, going to various outdoor activities, and ice skating.

Pranav Ravichandran
Pranav Ravichandran is from Glen Allen, Virginia and graduated from Mills E. Godwin High School’s Specialty Center for Science, Math, and Technology. He is graduating with a Bachelor of Science with Specialization in Biochemistry degree.
Pranav has been a member of the Perez-Reyes lab in the Department of Pharmacology since spring semester of his first year. He has worked on a variety of different projects and has since focused his time on testing a novel model of epilepsy. His research has focused on stimulating mice and testing novel gene therapies to cure epilepsy symptoms.
Outside of his research, Pranav volunteered at the Charlottesville-Albemarle Rescue Squad as an EMT for a large portion of his time. He has worked as a TA for CHEM 2411 and as an assistant to set up the labs in both CHEM 2411 and CHEM 2421. He enjoys spending his free time playing video games or generally tinkering around and building things. After graduation, he will be matriculating to medical school.

Amirus Saleheen
Amirus Saleheen has joined the Department of Chemistry as a Postdoctoral Research Associate in the Pompano lab in August 2020. Amirus obtained his B.S. in Applied Chemistry & Chemical Engineering at the University of Dhaka, Bangladesh. He received his Ph.D. in Chemistry at the University of Tennessee, Knoxville. His Graduate work consisted of developing a novel microfluidic chip to mimic rotary tube culture of rodent brain slices to improve the temporal resolution of downstream perfusate analysis. He also developed a smartphone based quantitative analysis method of Epinephrine in expired and discolored commercial auto-injectors to measure drug availability. In the Pompano lab, Amirus will be engaged in robust fluidic control and stimulation of a lymph node-on-a-chip.

Tochukwu Ozulumba
Tochukwu Ozulumba has joined the Department of Chemistry as a Research Associate in the Pompano Lab. Tochi obtained her BSc and MSc degrees in Biochemistry at the University of Nigeria and then completed a PhD in Biomaterials Science at the University of Brighton, United Kingdom. Her PhD project investigated the potential of two-dimensional graphene and titanium carbide MXene nanomaterials to target biological toxins and the incorporation of these materials into composite systems for use in medical devices. In the Pompano lab, Tochi will be working on controlling immune cell interactions with biomaterials in a spatially organized microphysiological model of a human lymph node.

Yin Zhouyang
Yin Zhouyang obtained his Bachelor's degree in chemistry from Nanjing University in China. Then he came to U.S. and received his Ph.D. degree in inorganic chemistry at Brown University under the guidance of Professor Shouheng Sun. He joined Professor Sen Zhang's group in 2020 as a postdoc associate with a focus on electro-catalysis and biomass conversion.

Lavoisier Akoolo
Lavoisier Akoolo joined the Pompano Lab as an immunity research scientist in October. He trained and worked as a veterinarian at the University of Nairobi in Nairobi, Kenya before going on to Texas A&M University in College Station, Texas, where he graduated with a PhD in Veterinary Pathology in 2015. Lavoisier went on to Rutgers New Jersey Medical School for post-doc training in microbial pathogenesis from 2015 to 2020. He has several publications in microbiology and is currently studying the use of live slices of lymph node tissue as a model of immunity in the Pompano Lab.

Jason J. Chruma
Education
B.S. with Honors – University of Arizona, 1996
Ph.D. – University of Pennsylvania, 2002
Professor Chruma is teaching organic chemistry lecture and laboratory courses, with a particular focus on organic laboratory for non-majors/minors on a pre-health career track. Over the past two decades, he established a highly collaborative international research portfolio in organic synthesis. Independent research interests involved the development of new methods for the synthesis of biologically-active natural products, drug-like scaffolds, and luminescent small molecules, with a particular focus on using 2-azaallyl anions as imine umpolungs and super-electron-donors. Moreover, Prof. Chruma is an internationally-decorated instructor in organic chemistry, with over 10 teaching excellence awards including the 2018 Tang Lixin Teaching Masters Award from Sichuan University in Chengdu, China.
Jason J. Chruma earned his B.S. with Honors in Chemistry from the University of Arizona (1996) and his Ph.D. in Chemistry from the University of Pennsylvania (2002). After completing a NIH postdoctoral fellowship at Columbia University, Mr. Chruma joined the faculty at the UVA Department of Chemistry in 2005 as an Assistant Professor. In 2012, Prof. Chruma transitioned to the College of Chemistry at Sichuan University (SCU) where he served from 2012–2020 as a Professor and Assistant to Dean on International Affairs; Jason still co-directs a research program and (up through July 2022) holds a part-time summer position at SCU as a high-level international faculty. Finally, Mr. Chruma is an active member of the American Chemical Society (ACS), serving as the founding Secretary and second Chair of the Southwestern China International Chemical Sciences Chapter of the ACS and as an Associate on the International Activities Committer (IAC) of the ACS.
Selected Recent Publications
Nickel-Catalyzed Decarboxylative Generation and Asymmetric Allylation of 2-Azaallyl Anions. C. Liu, C. Deng, H. Yang, X. Qian, S. Tang, M. Poznik, J. J. Chruma, J. Org. Chem. 2019, 15, 10102–10110. DOI: 10.1021/acs.joc.9b01293; PMID: 31328915.
Palladium-Catalysed Decarboxylative Generation and Regiodivergent Prenylation of 2-Azaallyl Anions. X. Wang, X. Zeng, Q. Lin, M. Li, P. J. Walsh, J. J. Chruma, Adv. Synth. Catal. 2019, 361, 3751–3757. DOI: 10.1002/adsc.201900491.
Palladium-Catalyzed Decarboxylative Generation and Propargylation of 2-Azaallyl Anions. S. Tang, W. Wei, D. Yin, M. Poznik, J. J. Chruma, Eur. J. Org. Chem. 2019, 3964–3978. DOI: 10.1002/ejoc.201900537.
2-Azaallyl Anions, 2-Azaallyl Cations, 2-Azaallyl Radicals, and Azomethine Ylides. S. Tang, X. Zhang, J. Sun, D. Niu, J. J. Chruma, Chem. Rev. 2018, 118, 10393–10457. DOI: 10.1021/acs.chemrev.8b00349; PMID: 30302999.
2-Azaallyl Anions as Light-Tunable Super-Electron-Donors: Coupling with Aryl Fluorides, Chlorides, and Bromides. Q. Wang, M. Poznik, M. Li, P. J. Walsh, J. J. Chruma, Adv. Synth. Catal. 2018, 360, 2854–2868. DOI: 10.1002/adsc.201800396.
Polyunsaturated Fatty Acid Amides from the Zanthoxylum genus – from Culinary Curiosities to Probes for Chemical Biology. J. J. Chruma, D. J. Cullen, L. Bowan, P. H. Toy, Nat. Prod. Rep. 2018, 35, 54–74. DOI: 10.1039/C7NO00044H; PMID: 29299588.

Blake Whitt
Blake Whitt joined the Department of Chemistry in August as a Research Associate in Dr. Marilyne Stains' lab. Blake earned his B.A. in Psychology at Arkansas Tech University, a Master's Degree in Behavioral Neuroscience at Michigan State University, and a Ph.D. in Science Education at the University of Georgia. His doctoral work examined the efficacy of various professional development programs designed to support university STEM faculty's adoption of evidence-based instructional practices. In the Stains lab, Blake will be investigating the influence of course coordination and department-level initiatives on instruction in undergraduate STEM.

John Zima
John Zima did research with Professor Ian Harrison where he used the Master Equation Solver for Multi-Energy Well Reactions (MESMER) in order to model the energetic relaxation of a photoexcited gas (e.g. methane) in a buffer gas (e.g. Argon) and then built a model for the energetic relaxation of the gas adsorbed on a metal surface using an analogy where the surface was effectively modelled as a bath gas.
John is an Echols Scholar and an international student from Austria. He has lived in Singapore, Switzerland and the UK before coming to UVA and is fluent in both English and German. During high school he played tennis and was interested in artificial intelligence, which led participating in Kaggle competitions. He is also interested in philosophy and attended the CTY summer program at Johns Hopkins University in the summer of 2016 for a course titled 'Logic: Principles of Reasoning'. John is doing the 3+1 Chemistry Program (and double majoring in economics) which means he will stay for another year, during which he will do research in the field of Astrochemistry with Professor Garrod, and graduate with a Masters degree in 2022. He then plans to pursue further graduate level education.

Julia Dressel
Julia is from Clifton, VA, graduating from Centreville High School. She is graduating from the University of Virginia with a B.S. in Chemistry with ACS Certification and a B.A. in Environmental Science.
Since January 2018, she’s worked with Dr. Charles Machan on transition metal catalysts for energy relevant transformations. In her first project, she worked with then graduate student Dr. Shelby Hooe to report the first highly efficient molecular chromium catalyst for the electroreduction of CO2 to CO, an important building block for many valuable synthetic chemicals and fuels. She then transitioned to an independent project by developing a similar nickel catalyst and evaluating it as a catalyst for the hydrogen evolution reaction, which can be used in carbon-neutral fuel cells. Her work has been recognized with the ACS Division of Inorganic Chemistry Award for Undergraduate Research.
Outside of the lab, Julia has been heavily involved in science education and outreach as a teaching assistant for CHEM 1820 and 2810 on the accelerated chemistry track, a board member of the ACS Student Chapter at UVA, and on the College Science Scholars Council Outreach Committee. She’s also continued her lifelong dance hobby with the University Dance Club.
After graduation, Julia will pursue a Ph.D. in Chemistry as a National Science Foundation Graduate Research Fellow at Stanford University where she plans to expand her understanding of clean energy relevant chemistry and continue her engagements in science education and outreach.

Hannah Ashe
Hannah grew up in Potomac, MD and graduated from Winston Churchill High School. She will be graduating from UVA with a Bachelor of Science in Chemistry with a Specialization in Biochemistry, with ACS certification and minors in Psychology and History.
Hannah joined the lab of Dr. Dan Gioeli in the Department of Microbiology, Immunology, and Cancer Biology in Fall 2019 after spending two years in Dr. Steven Zeichner's lab in the Department of Pediatrics. Hannah's projects with the Gioeli lab originally centered around prostate cancer. Her first project focused on the lncRNA, HULLK, and its impact on tyrosine phosphorylation in cell signaling pathways. She also first authored a review on the transcription factor, RUNX, and its role in castration resistant prostate cancer. For Hannah's DMP, she switched focuses to non-small cell lung cancer. She used immunohistochemical staining of lung cancer adenocarcinomas to elucidate the differences in immune modulatory protein expression in EGFR and KRAS mutant positive non-small cell lung cancer patients. Previous research has shown differential outcomes to immunotherapies based on mutation status in lung cancer, so she hopes her project will further our knowledge into why this is the case, and thereby improve treatment for patients.
Outside of the lab, Hannah is a huge fan of UVA basketball. She also enjoys shooting hoops with her roommate. Hannah has worked as a peer teacher for Intro Bio and as a TA for CHEM 1411. After graduating, Hannah will be working as a research technician in the Institute for Systems Genetics at NYU Langone Hospital in New York City. She eventually plans on applying to medical school.
Rebecca Schelling
Rebecca is from Westchester, NY and graduated from Horace Greeley High School. She is graduating from the University of Virginia with a B.A. in Chemistry, a B.A. in Psychology, and a minor in Biology.
Rebecca joined Dr. Ken Hsu's lab in the Department of Chemistry in January 2019. Her project focused on Transglutaminase 2-catalyzed serotonylation, a reaction which modifies scaffolding proteins and small G proteins for a wide range of downstream effects. She used activity-based protein profiling to work towards the first global profiling of serotonylation modification in the proteome, an important step for establishing potential sites for drug targeting.
Outside of lab, Rebecca is an avid member of HoosITS, UVA's broadway a cappella group, a tutor for student athletes, and the co-president of the Nu Rho Psi chapter at UVA. After graduation, Rebecca will be working as a postbac IRTA in the Laboratory of Molecular and Cellular Neurobiology at the NIMH in Bethesda, MD. She plans to apply to graduate school for pharmacology after her postbac.

Alyssa Montalbine
Alyssa is from Findlay, OH, graduating from Findlay High School. She is graduating from the University of Virginia with a B.S. in Chemistry with a Specialization in Biochemistry, ACS Certification, and ASBMB Certification, and Highest Distinction for her research dissertation.
Since January 2018, Alyssa has worked with Dr. Rebecca Pompano on developing microfluidic systems and bioanalytical assays for immunology research. In her time at UVA, she obtained three research grants and made substantial contributions to four separate research projects, several research publications, and one literature review. Her work has spanned four separate projects including optimizing the ninhydrin assay for quantifying hydrogel functionalization, assisting in the development of a lymph node organ-on-chip system, performing surface chemistry analysis for hydrophilic/hydrophobic microfluidic systems, and conceptualizing the design for a biomimetic ex vivo T cell cortex. Her work has been recognized with the ACS Division of Analytical Chemistry Award for Undergraduate Research. It has been Alyssa's greatest honor to work with Professor Pompano and the brilliant student researchers who have been a part of the Pompano Laboratory throughout the years.
Outside of research, Alyssa has been heavily involved in science education and outreach as the President of the ACS Student Chapter at UVA, a Teaching Assistant for CHEM 4410, and a collaborator with Dr. Laura Serbulea to develop online pre-lab modules for the advanced organic chemistry lab series. Alyssa was awarded the ACS Chapter Award for her dedication to serving the UVA Chemistry community. Alyssa has also been actively involved as a RA for Gooch-Dillard, a Co-Music Director for Hoos in the Stairwell Broadway Acapella, and a member of First-Year Players and CatholicHoos.
After graduation, Alyssa will pursue a Ph.D. in Biomedical Engineering as a National Science Foundation Graduate Research Fellow at the Georgia Institute of Technology. At Georgia Tech, Alyssa plans to specialize in Immunoengineering and Organ-on-Chip systems, while continuing to share her love for the lymph node with her fellow cohort. She would most especially like to thank Dr. Rebecca Pompano, Dr. Laura Serbulea, and Cindy Knight for being her greatest mentors and supports over the last four years. None of this would have been possible without them.

Alfred Burger
Dr. Alfred Burger was born in Vienna, Austria, in 1905 and received a Ph.D. degree from the University of Vienna in 1928. He was employed as a research chemist by the Hoffman-LaRoche Company before coming to the United States in 1929. He became a Research Associate at the Drug Addiction Laboratory of the National Research Council at the University of Virginia where he conducted research on the chemistry of opium alkaloids and the synthesis of morphine substitutes. In 1938, he joined the faculty of the Department of Chemistry at the University of Virginia. He taught organic and medicinal chemistry to over 4,500 students before retiring in 1970. Dr. Burger passed away December 30, 2000 at the age of 95.
His research activities with a staff of 40 graduate and 33 postdoctoral students included studies on the design and synthesis of analgesic, chemotherapeutic, and antidepressant drugs. One of his synthetic compounds was developed as a widely used clinical antidepressant under the name of tanylcypromine (Parnale). He was Chairman of the UVA Chemistry Department in 1962-63, a visiting professor in biochemistry at the University of Hawaii, and a lecturer at many American and foreign universities and industrial research departments. He was the first to bring female graduate students into the UVA Department of Chemistry.
In the 1950s, professor emeritus Alfred Burger of the University of Virginia defined the principles of medicinal chemistry when he wrote its first-ever textbook, Burger's Medicinal Chemistry and Drug Discovery, now in its sixth edition (2013). Burger also became the first editor of the Journal of Medicinal Chemistry at its foundation in 1958.
Dr. Burger received the Louis Pasteur Medal of the French Pasteur Institute, the Smissman Award of the American Chemical Society, and the Award in Medicinal Chemistry from the American Pharmaceutical Association. The ACS award in Medicinal Chemistry is named for him. He founded the Journal of Medicinal Chemistry in 1958 and served as its editor for 14 years. He also served as editor of Medicinal Chemistry Research and as Chairman of the ACS Division of Medicinal Chemistry.
Since 1980, the American Chemical Society awards the Alfred Burger Award in Medicinal Chemistry, sponsored by GlaxoSmithKline, in the amount of $5000 to recognize outstanding contributions to research in medicinal chemistry.
Professor Burger authored over 200 papers and numerous books, including his treatise on Medicinal Chemistry which is now in its sixth edition. His other books include Understanding Medications; Searching, Teaching & Writing-What Fun; Drugs & People; Drugs Affecting the Peripheral Nervous System; and Drugs Affecting the Central Nervous System.
At least seven talks were given by Dr. Burger at Virginia Section meetings. His presentations included:
“Micromolecular Chemistry: - January, 1937, Waynesboro “Some Problems of Chemotherapy” - November, 1940, Richmond “Chemotherapy Since 1940" - May, 1944, Richmond “Medical Chemistry Since the War” - February, 1948, Richmond “Medicinal Chemistry-Today and Tomorrow” - December, 1954, Richmond “Medicinal Chemistry-Its Problems and Hopes” - December, 1961, Richmond “How Do Medications Act and How Are They Discovered?” - March, 1992 - Farmville
Professor Burger played the violin and had a lifelong love of classical music. He was survived by his wife of 65 years, Frances Page Burger, and a daughter.
from - - American Chemistry Society. Virginia News & membership. February, 2001.

Greatness Olaitan

Djuro Raskovic

Ryan Johnson

Daniel Siela
Huiyuan Zhu
Education
B.Sc. in Chemistry University of Science and Technology of China, 2009
Ph.D. in Chemistry Brown University, 2014
-
Electrochemical CO2 conversion
-
Sustainable nitrogen cycling
-
Heterogeneous thermocatalytic reactions (e.g., acetylene semi-hydrogenation).
-
What are the catalytic active sites and how do they evolve under reaction conditions?
-
How to steer the reaction pathway toward desirable products via modulating active sites and their environments?
-
How to circumvent or ultimately, go beyond adsorption-energy scaling relations?
Selected Publications
2. Heterostructured Bi–Cu 2 S nanocrystals for efficient CO 2 electroreduction to formate
4. Harnessing strong metal–support interactions via a reverse route

Thomas Crowell
Thomas Irving Crowell III, of Charlottesville, Virginia, died at the age of 101, on Monday, October 10, 2022 at the Martha Jefferson House. He was born on July 9, 1921 in Glen Ridge, New Jersey, the oldest of four sons of Pauline Whittlesey Crowell and Thomas Irving Crowell Jr. In addition to his parents, he was preceded in death by his wife, Mary Wheat Crowell, R.N. (UVA 1950) and his brother, James W. Crowell, of Hanover, New Hampshire.
Tom is survived by two brothers, David A. Crowell of Concord, Massachusetts, and Kenneth L. Crowell and his wife, Marnie Reed Crowell of Sunset, Maine; his two daughters, Lesslie A. Crowell and Mary Allison Crowell, both of Charlottesville, Virginia; two grandsons, Gustave H. Rathe of Howardsville, Virginia, and Thomas C. Rathe and his wife, Sarah Cheshire, of Tuscaloosa, Alabama; sister-in-law, Deborah Crowell of Hanover, New Hampshire; and many nieces and nephews.
Tom attended public schools in his home town of Caldwell, New Jersey and The Putney School in Putney, Vermont. After graduating from Harvard University in 1943, he worked as a chemist on the Manhattan Project of the U.S. Army Corps of Engineers in New York City. At the end of World War II, he entered graduate school at Columbia University (Ph.D. 1948 in the laboratory of Professor Louis P. Hammett). While at Columbia he commuted to Bard College in Annandale-on-Hudson, New York to teach math. He joined the faculty of the University of Virginia in 1948 -but not before spending part of the summer riding a 3 speed bicycle from Manhattan to Quebec. At UVA he taught courses in organic and physical-organic chemistry and served as chairman of the chemistry department from 1957 to 1962.
At the University of Virginia's old Cobb Chemical Laboratory, Tom renovated a large corner room and used his glassblowing experience to make chemical apparatus. His research was in kinetics, the science of observing how fast substances react with each other when mixed, an important means of learning how new products are formed in nature. He and his students did early work using hot molten salts as solvents. He loved working in his laboratory more than sitting at his desk, and after retiring from UVA in 1984, he continued working in his home lab until he was 90. He was employed by Du Pont in Waynesboro, Virginia during the summer og 1951.
Tom was an enthusiastic amateur musician, studying piano from childhood to age 17, accompanying his brother and parents, all violinists. Later, at UVA, he played chamber music with many friends and was piano accompanist for the Gilbert and Sullivan operettas sponsored by the AAUW in Cabell Hall, and for the University Singers.
However, he always considered the French horn his primary instrument. He was president of the Harvard University Orchestra and played in the Massachusetts N.Y.A. Orchestra, the Columbia University Orchestra and the Virginia Symphony. He was a founding member of the University of Virginia Orchestra in 1949 and played in several wind quintets in Virginia, and earlier, in New York City.
Tom was a member of the Charlottesville Municipal Band in 1948 and played for several years with the Senior Center's Second Wind Band.
The study of nature brought Tom immense pleasure, and he spent much of his time hiking alone or with friends in New Hampshire, the Maine woods, and Virginia's Blue Ridge. In his later years, neighbors met him on his daily walks as recently as last spring. He also enjoyed playing tennis and sailing in the waters off Cape Cod. He was a member of the Unitarian Universalist Congregation of Charlottesville; the NAACP; Wednesday Music Club; and the American Chemical Society.
The family wishes to thank Tom's physicians, also the entire staff of the Martha Jefferson House for their kind and expert care.
A memorial service will be held at the Unitarian Universalist Congregation of Charlottesville, 717 Rugby Road, Charlottesville, on Saturday, November 5, 2022, at 3 p.m. Hill and Wood is handling arrangements.
In lieu of flowers memorial donations may be made to Southern Environmental Law Center, 120 Garrett Street, Suite 400, Charlottesville, VA 22902, or to the Martha Jefferson House, 1600 Gordon Avenue, Charlottesville, VA 22903.
Published by Daily Progress from Oct. 16 to Oct. 23, 2022.

Michelle Personick
Education
B.A. Middlebury College, 2009
Ph.D. Northwestern University, 2013
Postdoctoral Associate, Harvard University, 2013-2015
The Personick Group is advancing the state of the art in the synthesis of precise nanomaterials and is using these precision materials to define catalytic structure-function relationships at an elevated level of mechanistic detail. Through this combination of materials synthesis innovations and fundamental studies of catalytic reactivity, we aim to develop principles for the predictive design of catalyst materials that will enable key advances in technology for sustainable energy generation and chemical synthesis. Our research takes place at the interface between inorganic chemistry, materials science, and chemical engineering.
Innovation in the use of energy resources is a pressing contemporary challenge. Addressing this need requires the more efficient use of current petroleum-based energy resources as precursors and fuels, along with increased utilization of alternative energy resources, including biomass and natural gas, and the valorization of readily available but chemically stable potential feedstocks, such as carbon dioxide. Enhancing the viability of alternative energy generation technologies, such as fuel cells, is also required to transform the overall energy landscape. A major prerequisite in meeting these critical challenges in energy and sustainability is a need for the design and synthesis of new highly active and selective catalytic materials.
We are developing materials-generalizable chemical tools for controlling the structure, composition, and surface ligand environment of metal nanoparticles at the atomic scale. For example, we have designed a series of approaches for achieving fine control over the location and relative concentration of individual metals in bimetallic materials, which is essential for tuning catalyst performance. Our group has also pioneered an innovative methodology for using fundamental electrochemical measurements in combination with electrochemically-driven nanoparticle growth in a well-controlled chemical environment to understand redox transformations and the evolution of materials under the complex conditions of colloidal nanoparticle synthesis. Importantly, the technique is one of the only available tools for the in situ collection of data regarding the chemical processes that drive metal nanoparticle formation. In addition to answering key questions about nanoparticle growth, we are using this electrochemical approach to synthesize nanostructured electrodes from earth-abundant metals for energy-relevant electrocatalytic processes such as the conversion of carbon dioxide to fuels.
These nanomaterials provide a platform for structure-activity studies of catalytic reactions at the intersection of idealized model surfaces and working catalysts, thereby facilitating catalyst design from fundamental principles. We study these same highly precise “nanoscale model surfaces” under realistic catalytic conditions and in well-controlled chemical environments, tracking structure-function relationships resulting from clearly defined modifications of surface atomic arrangement, elemental composition, and the presence of molecular adsorbates. We use a variety of spectroscopic and microscopic techniques—including infrared spectroscopy, electron microscopy, x-ray photoelectron spectroscopy, and x-ray absorption spectroscopy—to understand the structure and dynamics of catalytic active sites. With this platform, we can test and validate predictions from experimental and computational surface science under working catalytic conditions, creating a feedback loop that facilitates directed catalyst design for a variety of applications. One current focus is selective oxidation and hydrogenation reactions relevant to the production of sustainable high-energy-density fuels and bio-based chemicals from biomass feedstocks.
We are using visible light to achieve reaction selectivity that is not possible with purely thermal reaction chemistry in both materials synthesis and catalytic transformations. Noble metal nanoparticles, particularly those of silver and gold, have unique properties that arise upon illumination with visible light. Energy from this “plasmonic” excitation can be used to control reactivity at the surfaces of these materials. We are employing this non-thermal, light-driven chemistry to selectively accelerate kinetically slow metal ion reduction processes and overcome challenges in the synthesis of bimetallic nanoparticles. We are also exploring plasmon-mediated catalytic reactions at crystalline defects and bimetallic interfaces—including core-shell, core-satellite, and dilute bimetallic surface architectures. Reactions relevant to air quality remediation, such as the oxidation of volatile organic compounds, are one target of this work. In addition, we are using plasmonic excitation as a tool for actively modulating the binding strength of weakly-adsorbed reaction intermediates on nanoscale metal surfaces to tailor selectivity in challenging molecular transformations relevant to sustainable chemical production.
Selected Recent Publications
Argento, G. M.; Judd, D. R.; Etemad, L. L.; Bechard, M. M.; Personick, M. L. “Plasmon-Mediated Reconfiguration of Twin Defect Structures in Silver Nanoparticles.” J. Phys. Chem. C. 2023, 127, 3890-3897.
McDarby, S. P.; Personick, M. L. “Potential-Controlled (R)Evolution: Electrochemical Synthesis of Nanoparticles with Well-Defined Shapes.” ChemNanoMat 2022, 8, e202100472.
McDarby, S. P.; Wang, C. J.; King, M. E.; Personick, M. L. “An Integrated Electrochemistry Approach to the Design and Synthesis of Polyhedral Noble Metal Nanoparticles.” J. Am. Chem. Soc. 2020, 142, 21322-21335.
Habib, A.†; King, M. E.†; Etemad, L. L.; Distler, M. E.; Morrissey, K.; Personick, M. L. “Plasmon-Mediated Synthesis of Hybrid Silver-Platinum Nanostructures.” J. Phys. Chem. C 2020, 124, 6853-6860. †Authors contributed equally.
King, M. E.; Kent, I. A.; Personick, M. L. “Halide-Assisted Metal Ion Reduction: Emergent Effects of Dilute Chloride, Bromide, and Iodide in Nanoparticle Synthesis.” Nanoscale 2019, 11, 15612-15621.
Robertson, D. D.; Personick, M. L. “Growing Nanoscale Model Surfaces to Enable Correlation of Catalytic Behavior Across Dissimilar Reaction Environments.” Chem. Mater. 2019, 31, 1121-1141.
King, M. E.; Personick, M. L. “Iodide-Induced Differential Control of Metal Ion Reduction Rates: Synthesis of Terraced Palladium-Copper Nanoparticles with Dilute Bimetallic Surfaces.” J. Mater. Chem. A 2018, 6, 22179-22188.
Stone, A. L.; King, M. E.; McDarby, S. P.; Robertson, D. D.; Personick, M. L. “Synthetic Routes to Shaped AuPt Core-Shell Particles with Smooth Surfaces Based on Design Rules for Au Nanoparticle Growth.” Part. Part. Syst. Charact. 2018, 35, 1700401.
Robertson, D. D.; King, M. E.; Personick, M. L. “Concave Cubes as Experimental Models of Catalytic Active Sites for the Oxygen-Assisted Coupling of Alcohols by Dilute (Ag)Au Alloys.” Top. Catal. 2018, 61, 348-356.
King, M. E.; Personick, M. L. “Defects by Design: Synthesis of Palladium Nanoparticles with Extended Twin Defects and Corrugated Surfaces.” Nanoscale 2017, 9, 17914-17921.
King, M. E.; Personick, M. L. “Bimetallic Nanoparticles with Exotic Facet Structures via Iodide-Assisted Reduction of Palladium.” Part. Part. Syst. Charact. 2017, 34, 1600422.
See more (ORCiD)
See more (Google Scholar)
Awards and Honors
2018 Young Investigator Program Award, Army Research Office
2018 Emerging Investigator, Journal of Materials Chemistry A, The Royal Society of Chemistry
2016 Victor K. LaMer Award, ACS Division of Colloid and Surface Chemistry

Jetze J. Tepe
Education
B.S. Jacksonville University, 1992
Ph.D. University of Virgnia, 1998
Postdoctoral Fellow, Colorado State University, 1998-2000
Organic and Medicinal Chemistry
Research in our lab is an interdisciplinary blend of synthetic organic chemistry, medicinal chemistry, and pharmacology.
Total Synthesis of Natural Products
Natural products represent a highly diverse and structurally complex natural library of compounds with remarkable biological and medicinal properties. Our lab develops new chemical methodologies to prepare natural products and natural product mimics with the goal of unraveling new mechanisms of protein regulation and their medicinal implications.
Targeting the Undruggable
Our research focus is on targeting “undruggable proteins” using small molecules to treat cancer and neurodegenerative disorders. We are one of the first to explore the enhancement of proteolytic degradation of classical undruggable proteins, such as c-Myc, a-synuclein, tau, amyloid-b, and others, by activation of the 20S proteasome. As part of this effort, our lab develops new heterocyclic reactions to synthesize complex marine sponge metabolites and their drug-like derivatives. The natural products and their drug-like derivatives are subsequently evaluated and optimized for their clinical significance in vitro, in cell culture and in in vivo disease models.
Representative Publications
- George, Dare; Tepe, Jetze J. Total Synthesis of Nagelamide W. Journal of Organic Chemistry, 2023, 88, 13, 9306–9312.
- Savelson, Evan; Tepe, Jetze J. Accessing Highly Oxidized Imidazolidinone Cores via a Curtius Rearrangement: Total Synthesis of Colensolide A. Organic Letters 2023, 25, 3698–3701.
- Staerz, Sophia S.; Lisabeth, Erika M.; Njomen, Evert; Dexheimer, Thomas S.; Neubig, Richard R.; Tepe, Jetze J. Development of a Cell-Based AlphaLISA Assay for High-Throughput Screening for Small Molecule Proteasome Modulators. ACS Omega, 2023, 8, 15650–15659.
- Vanecek, Allison, S.; Mojsilovic-Petrovic, Jelena; Kalb, Robert, G.; Tepe, Jetze J. Enhanced Degradation of Mutant C9ORF72-Derived Toxic Dipeptide Repeat Proteins by 20S Proteasome Activation Results in Restoration of Proteostasis and Neuroprotection, ACS Chemical Neuroscience 2023, 14, 1439–1448.
- Hubbell, Grace E.; Tepe, Jetze J. Rh(III)-catalyzed C-H activation/annulation of benzo- hydroxamates and 2-imidazolones: access to urea-fused-dihydroisoquinolone scaffolds reminiscent of pyrrole-alkaloid natural products, Organic Letters, 2022, 24, 6740–6744.
- Njomen, Evert; Vanecek, Allison; Lansdell, Theresa A.; et al., Small Molecule 20S Proteasome Enhancer Regulates MYC Protein Stability and Exhibits Antitumor Activity in Multiple Myeloma, Biomedicines 2022, 10, 938.
- Keel, Katarina L.; and Tepe, Jetze J. Total Synthesis of Nortopsentin D via a Late-Stage Pinacol-like Rearrangement, Organic Letters, 2021, 23, 5368-5372.
- Fiolek, Taylor J.; Keel, Katarina L. and Tepe, Jetze J. Fluspirilene analogs activate the 20S proteasome and overcome proteasome impairment by intrinsically disorder protein oligomers, ACS Chemical Neuroscience 2021, 12, 1438–1448.
See more (Google Scholars) https://scholar.google.com/citations?user=_DtCKxcAAAAJ&hl=en
Awards and Honors
- William J. Beal Outstanding Faculty Award, MSU, 2022
- MSU Inventor of the Year Award, 2022
- AbbVie Innovation Midwest Award, 2021
- Outstanding Faculty Award, MSU College of Natural Sciences, 2021
- International Myeloma Foundation, Brian D. Novis Award, 2019 & 2013
- Multiple Myeloma Research Foundation Award, 2010 & 2008
- American Cancer Society Research Scholar Award, 2003-2008
Jason Bates
Education
B.S., University of Kansas, Chemical Engineering, 2014
Ph.D., Purdue University, Chemical Engineering, 2019
Postdoctoral, University of Wisconsin–Madison, Chemistry, 2020–2023
Jason received his B.S. in Chemical Engineering at the University of Kansas in 2014 and his Ph.D. in Chemical Engineering at Purdue University in 2019. At Purdue, he studied the fundamental kinetic contributions of solvation and active site structure to dehydration reactions relevant to biomass upgrading using structurally well-defined Lewis acid and Brønsted acid zeolite catalysts. He was an NIH postdoctoral fellow at the University of Wisconsin–Madison in the Department of Chemistry, where he bridged the fields of thermal and electrocatalysis through studies of catalysts consisting of atomically dispersed metals incorporated into nitrogen-doped carbon.
In the Bates research group, we synthesize catalysts with well-defined structures and use quantitative kinetic measurements and characterizations of their active centers to elucidate structure–reactivity relationships. Our studies are enriched by a network of collaborations (e.g., theorists) and advanced characterization tools at national laboratories. We apply our approach to study catalytic technologies relevant for decarbonization of the energy and chemical industries.
Selected Publications
Chemical Kinetic Method for Active-Site Quantification in Fe-N-C Catalysts and Correlation with Molecular Probe and Spectroscopic Site-Counting Methods. Journal of the American Chemical Society 2023, 145, 26222–26237. Abstract
Molecular Catalyst Synthesis Strategies to Prepare Atomically Dispersed Fe-N-C Heterogeneous Catalysts. J. Am. Chem. Soc. 2022, 144, 18797–18802. Abstract
Heterogeneous M-N-C Catalysts for Aerobic Oxidation Reactions: Lessons from Oxygen Reduction Electrocatalysts. Chem. Rev. 2023, Doi: 10.1021/Acs.chemrev.2c00424. Abstract
Chemical and Electrochemical O2 Reduction on Earth-Abundant M-N-C Catalysts and Implications for Mediated Electrolysis. J. Am. Chem. Soc. 2022, 144, 922–927. Abstract
Kinetic Effects of Molecular Clustering and Solvation by Extended Networks in Zeolite Acid Catalysis. Chem. Sci. 2021, 12, 4699–4708. Abstract
Structure and Solvation of Confined Water and Water-Ethanol Clusters within Microporous Brønsted Acids and their Effects on Ethanol Dehydration Catalysis. Chem. Sci. 2020, 11, 7102–7122. Abstract
Distinct Catalytic Reactivity of Sn Substituted in Framework Locations and at Defect Grain Boundaries in Sn-Zeolites. ACS Catal. 2019, 9, 6146–6168. Abstract